The conspiracy-oriented group Architects and Engineers for 9/11 Truth claims there is lots of evidence the the World Trade Center towers were brought down by a complex sequence of pre-planned demolition charges using a silent yet powerful classified explosive substance capable of precisely cutting steel beams in half and then throwing them hundreds of feet in the air. They call this "nanothermite".
An obvious problem with this theory is the huge fires that burned for an hour before the buildings collapsed. Why didn't the fires set off the nanothermite?
AE911 attempts to answer this in their FAQ, mentioning the ignition of nanothermite in the detailed answer to FAQ #2:
The linked article continues:
FAQ #2: WHAT ABOUT THE PLANES THAT SLAMMED INTO THE TWIN TOWERS? WOULDN’T THEY HAVE DISTURBED THE DEMOLITION DEVICES?
Some of the demolition devices were undoubtedly disturbed by the plane impacts, but not enough to prevent the rest of the devices from performing adequately. Even the NIST report states that the collapse of the North Tower started on a floor with fairly minor structural damage. A more detailed answer is available here.
So the argument is that the fires were simply not hot enough to ignite the thermite.
Second, a demolition using advanced nano-thermite material (which has been identified in the World Trade Center dust) may help to explain why the fires started by the planes did not set off explosive devices. As explained by Dr. Steven Jones:
“It is important to note that initiating the thermite reaction requires temperatures well above those achieved by burning jet fuel or office materials — which is an advantage of using thermite charges over conventional monomolecular explosives such as TNT, RDX and PETN. Below is a photograph of an experiment performed by the author and colleagues at BYU in which a sample of thermite was heated to orange-hot temperature (about [927°C] 1700ºF ). We demonstrated that the thermite reaction would not ignite at this high temperature. Later, the thermite reaction was triggered by burning a magnesium strip in contact with the thermite...."
The article linked by "identified in the World Trade Center dust" goes on to say:
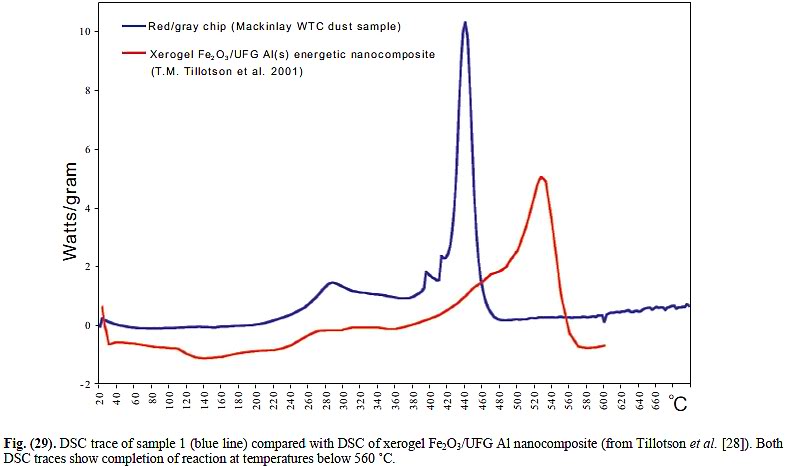
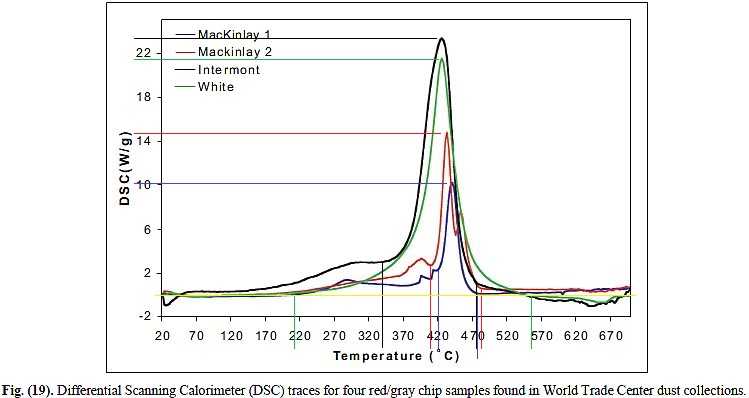
Hence AE911 is making contradictory claims. They say they found nanothermite in samples of dust that ignites at 430°C, but simultaneously claim that the nanothermite would have survived the fires because it does not ignite at 927°C, more than twice as hot.
Iron oxide and aluminum are the ingredients of classic thermite, an incendiary that burns unusually hot at approximately 4500°F, producing aluminum oxide and molten iron. The carbon content of the matrix indicates the presence of an organic substance.
When the red/gray chips were heated to about 430° C. (806° F.), they ignited, releasing relatively large amounts of energy very fast. This behavior matches “fairly closely an independent observation on a known super-thermite sample”
Last edited: