A little digging around for another thread brought up something interesting in regard to Gary Nolen's analysis of the Ubatuba sample. This is a bit of speculation I'll admit upfront, but as the information concerning Nolan's samples seems to be almost non-existent one tries for a bit of reasoned speculation.
Recall from the overly long OP (sorry), Nolen claimed to have a possible piece of meta-material from a UFO that supposedly crashed in the Brazilian village of Ubatuba:
External Quote:
One of the materials from the
so called Ubatuba event [a UAP event in Brazil], has extraordinarily altered isotope ratios of magnesium. It was interesting because
another piece from the same event was analyzed in the same instrument at the same time. This is an extraordinarily sensitive instrument called a nanoSIMS - Secondary Ion Mass Spec. It had perfectly correct isotope ratios for what you would expect for magnesium found anywhere on Earth. Meanwhile, the other one was just way off. Like 30 percent off the ratios. The problem is there's no good reason humans have for altering the isotope ratios of a simple metal like magnesium. There's no different properties of the different isotopes, that anybody, at least in any of the literature that is public of the hundreds of thousands of papers published, that says this is why you would do that. Now you can do it. It's a little expensive to do, but you'd have no reason for doing it.
https://www.vice.com/en/article/n7n...nalyzing-anomalous-materials-from-ufo-crashes
Nolen's claim is that there were 2 sample of magnesium, one was "normal" but the other one had strange isotopes.
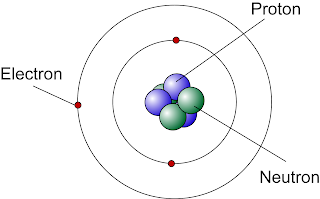
I'm no chemist, but isotopes are pretty easy to understand. For those that do, just skip ahead or feel free to correct me as needed here. Each element is made up of atoms. Atoms have + charged protons and non-charged neutrons bonded together in a nucleus with - charged electrons orbiting the nucleus, usually simplified into a diagram like this:
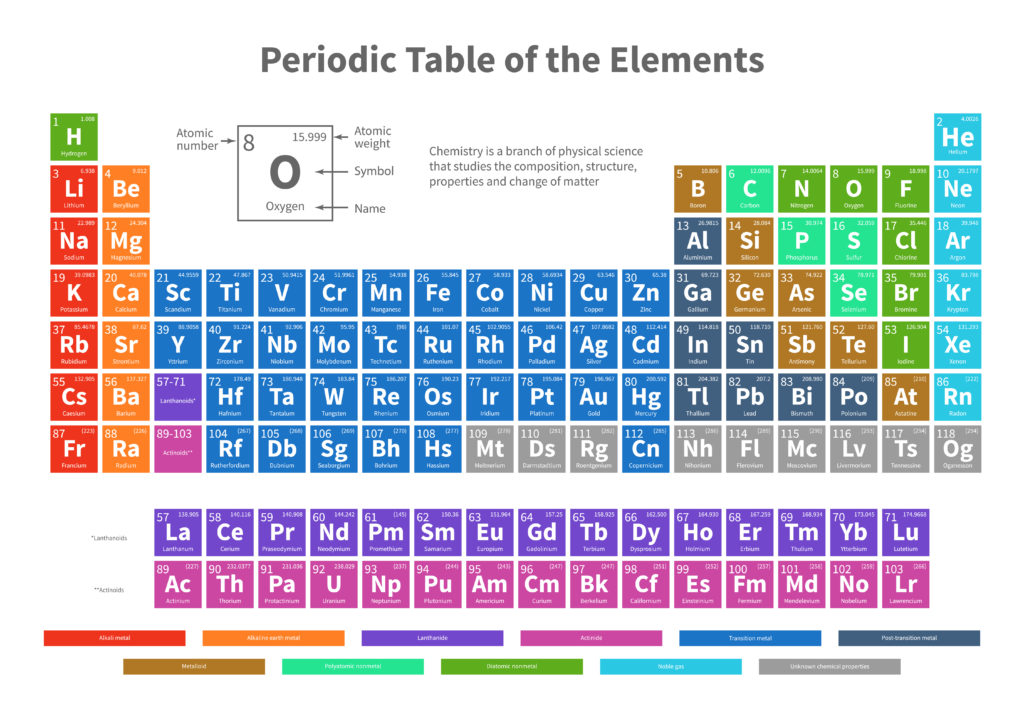
An elements atomic number and place on the periodic table is determined by the number of electrons and protons in the atom. They are always the same, so in the periodic table below, Oxygen is used as an example for reading the table. Oxygen's atomic number is 8, so it has 8 protons and 8 electrons. Atoms
usually have a similar number of neutrons, but not always. When an atom has a different number of neutrons from its atomic number, it's called an isotope.
Some isotopes are stable, that is they stay the way they are, and some are unstable, meaning they decay or give up neutrons until they have a stable amount of them. One of the well-known uses of this is in Carbon 14 dating.
On the periodic table above Carbon is C and has an atomic number of 6, so it has 6 electrons and 6 protons. It also has a number of stable isotopes including Carbon 12, that has 12 neutrons in its nucleus. Carbon 14, with 14 neutrons is radioactive, that is it decays or gives up neutrons until it has 12 and becomes stable. The known time that this takes is used in dating, which I'm not going to get into here.
Magnesium has an atomic number of 12 and is Mg on the periodic table. The interview with Nolen doesn't specify which isotopes of Mg were normal and which were unusual, just that some were.
Also in the interview, Nolen never says were he got the samples, so bear with me here as I try to create a hypothetical provenance for the Ubatuba samples he was testing.
In 2001 Peter Sturrock of Stanford University, the same Stanford that Nolen is at, conducted yet another analysis of the Ubatuba samples, and gives a history of them and how he got them in a paper he wrote for The Journal of Scientific Exploration a somewhat fringy journal that is claimed to be peer reviewed. External content below from here unless noted otherwise:
https://www.scientificexploration.org/docs/15/jse_15_1_sturrock.pdf
To start with, the samples first show up in Brazil. After they were sent the newspaper gossip columnist Sued, he gave them to a Dr. Fontes:
External Quote:
Fontes received from Sued three specimens that he refers to as "Samples 1, 2 and 3." Photographs of Samples 2 and 3 are reproduced as figure 1 of Fontes (1962). Their lengths were about 1/4 inch and 3/4 inch, respectively. Sample 1 was never photographed.
Then bits and pieces were giving to various Brazilian groups with Fontes keeping some of them:
External Quote:
Sample 1 was then divided into several pieces. Two were left with the laboratory, and Fontes retained the rest (together with Samples 2 and 3).
External Quote:
On November 4, 1957, Fontes gave one of the remaining pieces of Sample 1 to Major Roberto Caminha of the Brazilian Army, who had the specimen analyzed at the Military Institute of Technology
Then the samples were transferred to Coral Lorenzens and her husband, the founders and heads of APRO, a civilian UFO group similar to NICAP and MUFON:
External Quote:
In late 1957, Fontes conveyed the remaining piece of Sample 1, and also Samples 2 and 3, to the Lorenzens at APRO.
In 1967 the Lorenzens gave some of the samples to the Condon group which gave them to Craig:
External Quote:
In 1967, the Lorenzens contacted Dr. Edward U. Condon, who was serving as director of the Colorado Project, to examine UFO evidence under contract with the U.S. Air Force. Analysis of the Brazil magnesium specimen was assigned to Dr. Roy Craig, who has given a narrative account of his experiences with the Colorado Project (Craig, 1995). The results of his investigations into the Brazil magnesium are summarized in the Condon Report (Condon & Gillmor, 1969, pp. 94–97).
Craig had it tested, which we'll come back to below:
External Quote:
Craig was advised that the most sensitive test for impurities would be neutron activation analysis. He therefore arranged to take a specimen of the Brazil magnesium and (for comparison) a specimen of the Dow triply sublimed magnesium to the Alcohol, Tobacco, and Firearms (ATF) Laboratory in Washington, D.C. This visit took place on February 5, 1968, and the specimen was analyzed by Mr. Maynard J. Pro, whose report on the analysis was mailed to Craig on February 29, 1968.
Without getting too into the weeds, Sturrock got some of the samples and had them tested showing nothing unusual about their isotopic ratios:
External Quote:
With the kind cooperation of the Lorenzens, I was able to arrange for some analyses in California.
I was able to arrange for an isotopic analysis to be carried out at the Division of Geological and Planetary Sciences of the California Institute of Technology in Pasadena.
They were able to determine that, with an accuracy of 0.04% (400 ppm), there is no significant difference between the isotopic composition of the specimen I had provided and that of normal terrestrial magnesium that had been subjected to normal fractionation processes such as sublimation.
Sturrock ended up with most of the samples by the late '80s:
External Quote:
The Lorenzens kindly transferred ownership of the remaining specimens to me in 1987. It should be noted that, by that time, the association of the remaining specimens with the original three specimens had been completely lost. The specimens had not been carefully protected and tracked. In my discussions with the Lorenzens, I learned that two specimens were out on loan. One was in the possession of Mr. Robert Achzehnov of Costa Mesa, California; I subsequently retrieved this specimen from Mr. Achzehnov in 1986. The other has a more interesting history.
The more interesting sample had been the one tested by Roy Craig and ended up with someone else having been loaned out by the Lorenzens, inferring that Craig returned the sample to them. This is the one we're interested in and I'll call it the Craig sample going forward:
External Quote:
Mr. Harold Lebelson, a journalist, had expressed an interest in the Brazil magnesium in 1978. As a result, a specimen (the same specimen that had been analyzed by the Colorado Project) was given into his care by the Lorenzens.
He took this specimen to Professor Robert E. Ogilvie of the Metallurgy Department at the Massachusetts Institute of Technology (MIT).
He does some testing, and Ogilvie thought it maybe came from a "weld material" or a piece of welded Mg, such as might be in a jet or maybe early rockets attempting Earth orbit. Not a UFO. But then some nefarious guy took the sample:
External Quote:
However, on telephoning him, I was dismayed to learn that he no longer had the specimen. According to Ogilvie, Lebelson had telephoned him in 1984 and advised him that someone would visit Ogilvie on Lebelson's behalf to retrieve the specimen. Soon thereafter, a gentleman turned up at Ogilvie's laboratory and took possession of the magnesium. Ogilvie did not recall the person's name, did not check his credentials, and did not ask for a receipt. All that Ogilvie could remember about the visitor was that he said he was from the IBM plant in Fishkill, New York.
So, the Craig sample disappeared. However, Sturrock also points out that the Lorenzens didn't keep very good records of the samples:
External Quote:
Unfortunately, the Lorenzens did not keep a careful log of the specimens, so it is not possible at this time to identify that piece, or even to be sure that it is still part of the remaining specimens.
External Quote:
3 Since there had been no systematic tracking of specimens in the APRO files, it was convenient to adopt a new system of coding the various specimens when they were transferred from APRO to Stanford University. Specimens received from APRO were numbered SU-A, SU-B, etc. If a specimen was subdivided, its parts were coded SU-Ia, SU-Ib, etc.
Roy Craig was working for what's commonly called The Condon Committe in the '60s, as noted above, when he got a piece of the Ubatuba material from the Lorenzens. Some UFOlogist make a point of this, saying something like "the Ubatuba event must have been real because the US government looked into the material from it", as if testing the material was an endorsement of its origin. Never mind that the test didn't show much unusual.
Craig decided to use Neutron Activation analysis on a small piece of the sample, as Sturrock had said:
https://www.google.com/books/edition/UFOs/2FK54XizXNMC?hl=en&gbpv=1&pg=PA130&printsec=frontcover
External Quote:
Neutron activation analysis (NAA)
External Quote:
is the
nuclear process used for determining the concentrations of
elements in many materials. NAA allows discrete
sampling of elements as it disregards the chemical form of a sample, and focuses solely on atomic nuclei. The method is based on
neutron activation and thus requires a
source of
neutrons. The sample is bombarded with neutrons, causing its constituent elements to form radioactive isotopes.
External Quote:
To carry out an NAA analysis, the specimen is placed into a suitable irradiation facility and bombarded with neutrons. This creates artificial radioisotopes of the elements present.
https://en.wikipedia.org/wiki/Neutron_activation_analysis
NEA, creates new isotopes of the elements in the material being studied.
Craig took his sample to Washington DC:
https://www.google.com/books/edition/UFOs/2FK54XizXNMC?hl=en&gbpv=1&pg=PA130&printsec=frontcover
This might even be the lab pictured on the Wiki page for NEA close to the time Craig was there:
Just to be clear, the results weren't all that unique.
https://www.google.com/books/edition/UFOs/2FK54XizXNMC?hl=en&gbpv=1&pg=PA130&printsec=frontcover
And back then in the '50s and '60s the important point about the Ubatuba material was that its composition was very pure Mg and could not have been manufactured in '57. It's only since the '60s and into the '90s, after repeated analysis of the material has concluded otherwise, that the goal posts have been moved. Now it's not the purity of the material, but the strange isotopic ratios.
But what if the sample with the strange isotopic ratios is in fact the long lost Craig sample? The one that had been irradiated to create new and different isotopes?
I can't find any documents or statements from Nolan saying where he got the samples he tested. One assumes they are in the collection that Sturrock obtained from the Lorenzens and was kept at Stanford. None of Sturrock's analysis of the samples ever came up with unusual isotopic ratios, only Nolan's did. Or at least that's what he claims. Did a little piece of the Craig sample end up in the Stanford collection and was just never tested for isotopes? I don't know.