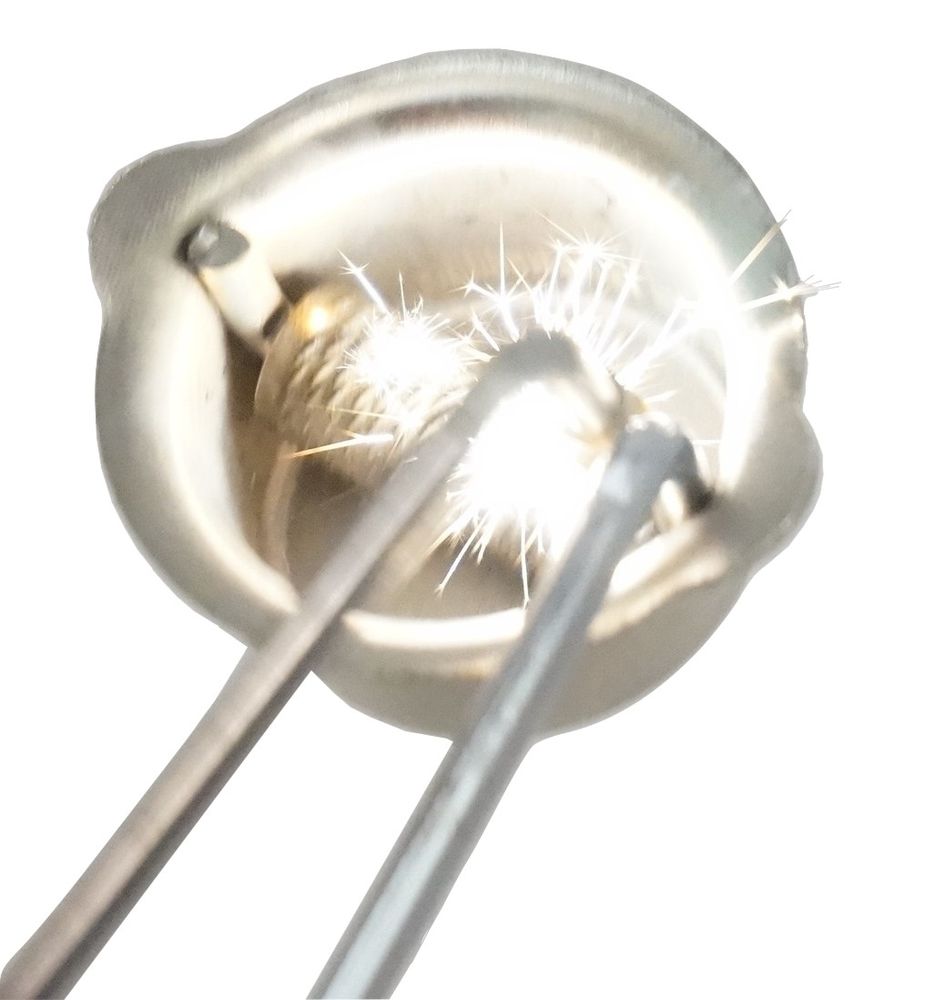
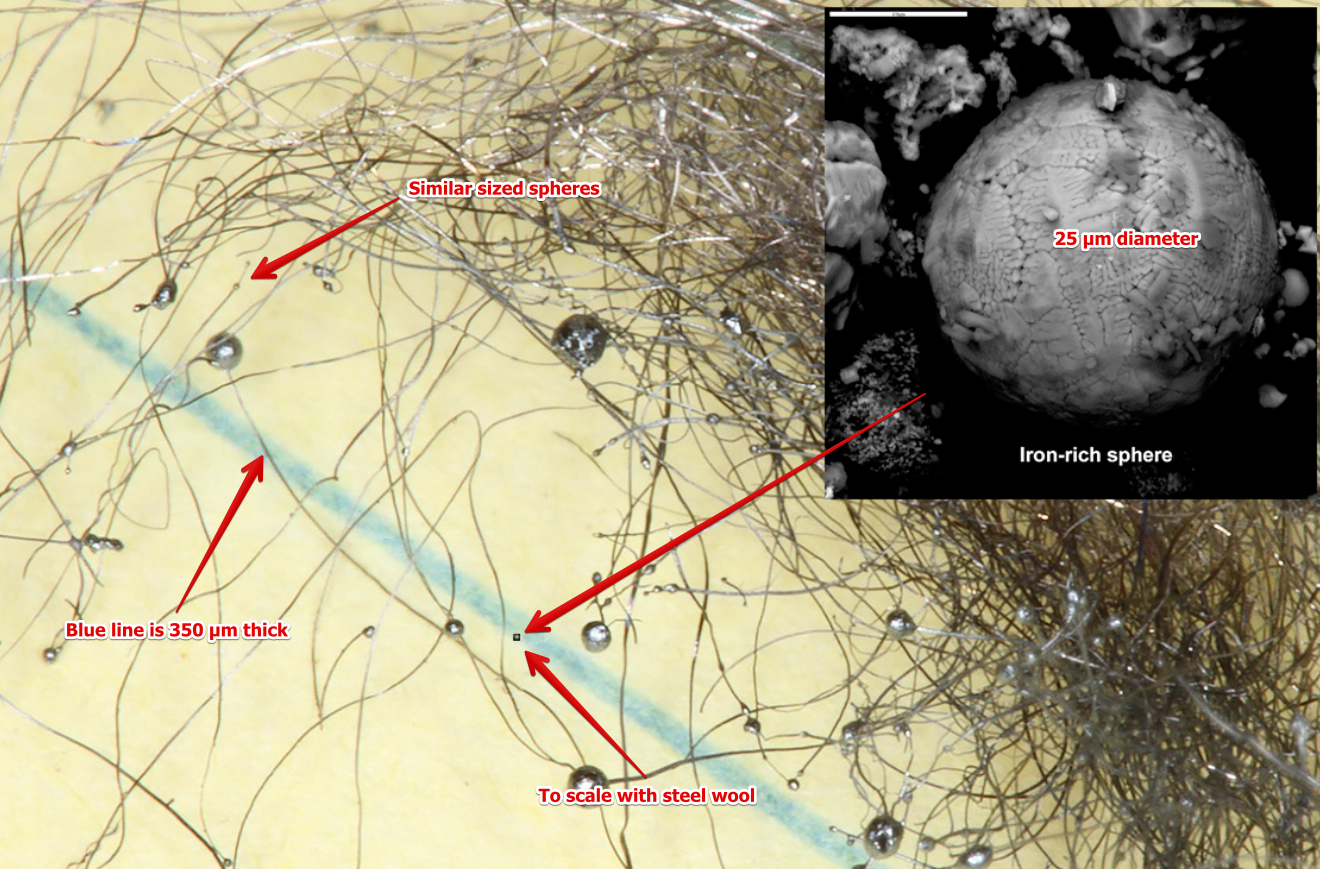
Iron rich microspheres can be made in various ways. In this thread I investigate some of them, and try to make some microspheres of my own.
Burning Methods (external ignition heat from flames)
- Burning Paint Chips #1. https://www.metabunk.org/posts/218647/
- Steel Wool #1: https://www.metabunk.org/posts/219558/
- Iron Filings #1: https://www.metabunk.org/posts/219574/
- Toner: https://www.metabunk.org/posts/219612/
- Steel Wool #2: https://www.metabunk.org/posts/219648/
- Burning Paint Chips #2: https://www.metabunk.org/posts/219649/
- Iron Powder 320 Mesh: https://www.metabunk.org/posts/225668/
- Iron Filings 50 Mesh: https://www.metabunk.org/posts/225672/
- *Pyrophoric iron: https://www.metabunk.org/posts/219592/
- Steel Wool #3 (physics investigation): https://www.metabunk.org/threads/what-happens-when-you-burn-steel-wool.10002/
Sparking methods (impact causing both scraping and ignition heat)
- Steel on steel impact. https://www.metabunk.org/posts/219454/
- Angle Grinder: https://www.metabunk.org/posts/219490/
- Bic Lighter: https://www.metabunk.org/posts/219555/
- Flint Striker: https://www.metabunk.org/posts/219584/
- Rust on aluminum impact: https://www.metabunk.org/posts/219689/
- 1600's Flint: https://www.metabunk.org/posts/219589/
Melting Methods (External Energy/Heat Melting steel/iron)
- Arc Welding #1 (Magnet capture): https://www.metabunk.org/posts/219499/
- Arc Welding #2 (Water capture): https://www.metabunk.org/posts/219888/
- *Oxy cutting
- *Thermal lance cutting
Chemical Reaction Method (chemical reaction with molten or vapor iron as a product)
- Thermite (Al + Fe2O3): https://www.metabunk.org/posts/219775/
* = Methods I've not personally tried
Last edited: