External Quote:
There is no evidence of individual elemental aluminum particles of any size in the red/gray chips
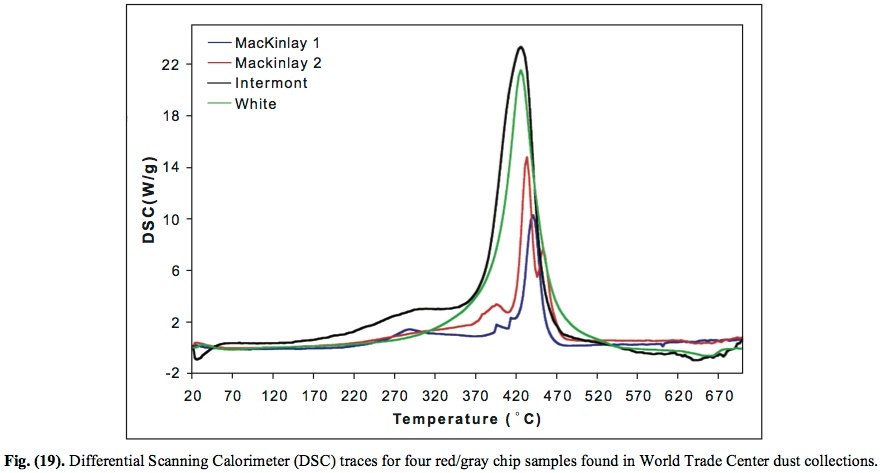
AND YET... And yet, the chips ignited at about 430C and left iron microspheres as a by-product.
Jazzy said:
It is quite obvious that they didn't.
It seems Dr. Millete and I are in disagreement with you on this one. The microscopy and spectroscopy seem to indicate a match for the material studied by Harrit, et. al. Not sure what grounds you have to dispute this, but as usual, this would be the time to back up your bare assertion with some kind of justification other than the letters after your name or a condescending tone.
Jazzy said:
Because they knew it wasn't reactive.
In a very real sense, this is equivalent to saying "they knew Harrit et.al. were completely fabricating their work." But, without actually saying that. It is also equivalent to saying "they knew it would ignite and didn't want to corroborate the work they were contracted to contradict."
Either way, it's a textbook example of poor scientific method. The whole point of laying out protocols for your experiment is so other people can do exactly the same thing. That way, if it does NOT turn out the same, the replicators are on solid ground to say "it's bunk." As it stands, Millete, et. al. did some sciency stuff, packed it up in a formal dress, and sold it to you and others as "good science." It is closer akin to a sales brochure.
The bottom line, no matter how you dress it up, is they did not replicate the study, and they did not find a match for the material in any paint they looked at, and you better believe they wanted to find a match.
Jazzy said:
The dust was a mixture of all sources. It couldn't be expected to be a match for any single source.
Yes, it could. If they found a sliver of paint, there is no doubt they would have matched it with the techniques they and the Harrit team were using. But they didn't. Any denial of this point is an indication of a poor understanding of the techniques at their disposal.
Jazzy said:
How about "incombustible molecules have no ignition point? That seems reasonable to me.
The whole point of the scientific method is to distinguish assumptions from reality. To go into the test assuming the substance will not ignite simply
isn't doing science.
Jazzy said:
They didn't ignite because there was no aluminum.
There was indeed aluminum. It wasn't elemental Al, true, but if it explodes, what difference does it make?
[...]
Just to be clear, let's have a mock dialogue to show you how unfounded your position is.
Jay: "the chips are thermitic."
Jazzy: "Well, do they ignite?"
Jay: "I didn't try to ignite them."
Jazzy: "Then how do you know they're thermitic?"
Jay: "I just know."
Of course, that's complete bs. You wouldn't accept that as any kind of rationale, would you? Let's try it the other way around:
Jazzy: "The chips are NOT thermitic."
Jay: "Do they ignite at 500C?"
Jazzy: "No, of course not!"
Jay: "So you tried to ignite them?"
Jazzy: "No."
Jay: "Why not?"
Jazzy: "Because they AREN'T THERMITIC!"
Jay: "How do you know?"
Jazzy: "I just know."
Again, complete bs. But this is EXACTLY the position you are maintaining. It's unfounded and it's not science.