You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Debunked: J. Marvin Herndon's "Geoengineering" Articles in Current Science (India) and IJERPH
- Thread starter Mick West
- Start date
skephu
Senior Member.
I have calculated the standard deviations of the element/aluminum ratios from the Moreno data, and they are huge.
Herndon calculated the 99% confidence intervals for the means, and performed a statistical test based on this. I can't reproduce what he did because he omits the rainwater data from his paper. But I can calculate the 99% confidence intervals for the means for the Moreno data. These are huge, and their lower limits are often negative, which doesn't make sense as you can't have a negative amount of an element, so I replaced the negative values by zero. Using this, the confidence intervals for the 7 "fingerprint elements" to aluminum ratios are below (Herndon says 8, but one is the Al/Al ratio which is 1 so makes no sense):
Ca/Al ratio: 0 to 15,773
Fe/Al ratio: 0.03 to 0.17
Mg/Al ratio: 0 to 28
S/Al ratio: 0 to 5,425
B/Al ratio: 0 to 18.5
Ba/Al ratio: 0 to 28
Sr/Al ratio: 0 to 271
So Herndon's method would identify any material with elemental ratios in these ranges as coal fly ash.
Interestingly, all the ranges are huge except that for iron. And here, there is a large discrepancy between the rain data and the coal fly ash data in Herndon's figure:
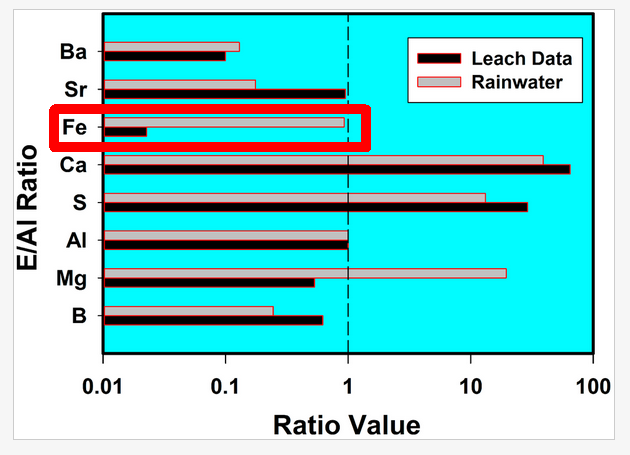
According to the figure, the iron to aluminum ratio is ~50 times as much in rain as in coal fly ash. The rain value is also well outside the 99% confidence interval shown above. Now of course if this ratio varies a lot in rain (we don't know because Herndon doesn't report the full rain data set) then the difference may not be significant.
Aside from the iron values, all the other ranges are so huge that "Herndon's method of coal fly ash recognition" would identify Earth's crust and virtually all rocks and all soils as made of coal fly ash.
Herndon calculated the 99% confidence intervals for the means, and performed a statistical test based on this. I can't reproduce what he did because he omits the rainwater data from his paper. But I can calculate the 99% confidence intervals for the means for the Moreno data. These are huge, and their lower limits are often negative, which doesn't make sense as you can't have a negative amount of an element, so I replaced the negative values by zero. Using this, the confidence intervals for the 7 "fingerprint elements" to aluminum ratios are below (Herndon says 8, but one is the Al/Al ratio which is 1 so makes no sense):
Ca/Al ratio: 0 to 15,773
Fe/Al ratio: 0.03 to 0.17
Mg/Al ratio: 0 to 28
S/Al ratio: 0 to 5,425
B/Al ratio: 0 to 18.5
Ba/Al ratio: 0 to 28
Sr/Al ratio: 0 to 271
So Herndon's method would identify any material with elemental ratios in these ranges as coal fly ash.
Interestingly, all the ranges are huge except that for iron. And here, there is a large discrepancy between the rain data and the coal fly ash data in Herndon's figure:
According to the figure, the iron to aluminum ratio is ~50 times as much in rain as in coal fly ash. The rain value is also well outside the 99% confidence interval shown above. Now of course if this ratio varies a lot in rain (we don't know because Herndon doesn't report the full rain data set) then the difference may not be significant.
Aside from the iron values, all the other ranges are so huge that "Herndon's method of coal fly ash recognition" would identify Earth's crust and virtually all rocks and all soils as made of coal fly ash.
Jay Reynolds
Senior Member.
Weidan Zhou has asked me to post this clarification here:
External Quote:I didn't know anything about the background or methods of this paper. And I had no idea that I was mentioned in the acknowledgements until yesterday.
Ah, another violation of MDPI policy for their papers:
With this sort of violation I'm wondering if he had a funder who wasn't mentioned. We may never know.External Quote:All sources of funding of the study should be disclosed. Please clearly indicate grants that you have received in support of your research work. Clearly state if you received funds for covering the costs to publish in open access. Note that some funders will not refund article processing charges (APC) if the funder and grant number are not clearly identified in the paper. Funding information can be entered separately into the submission system by the authors during submission of their manuscript. Such funding information, if available, will be deposited to FundRef if the manuscript is finally published. Authors must have obtained specific permission from individuals and institutions to mention their names in the Acknowledgements.
http://www.mdpi.com/journal/ijerph/instructions#preparation
Jay Reynolds
Senior Member.
Aside from the iron values, all the other ranges are so huge that "Herndon's method of coal fly ash recognition" would identify Earth's crust and virtually all rocks and all soils as made of coal fly ash.
Wangen(1981) noticed the same thing
Here's a little gold mine of data on elemental composition of atmospheric dust compared to coal fly ash, dust in California, China and various places, includes the Wangen data:
https://books.google.com/books?id=6...xaSCh1JVws0#v=onepage&q=wangen (1981)&f=false
Last edited:
Jay Reynolds
Senior Member.
An interesting development. Yesterday, Jeffrey Beale posted on Herndon's IJ article, and it is being critically examined by others......
http://scholarlyoa.com/2015/08/25/m...-swiss-chinese-publisher-mdpi/#comment-341469
I have commented referencing this thread which should prove interesting.

http://scholarlyoa.com/2015/08/25/m...-swiss-chinese-publisher-mdpi/#comment-341469
I have commented referencing this thread which should prove interesting.
Trailspotter
Senior Member.
Your comment has been published. I gave it thumb up.An interesting development. Yesterday, Jeffrey Beale posted on Herndon's IJ article, and it is being critically examined by others......
http://scholarlyoa.com/2015/08/25/m...-swiss-chinese-publisher-mdpi/#comment-341469
I have commented referencing this thread which should prove interesting.
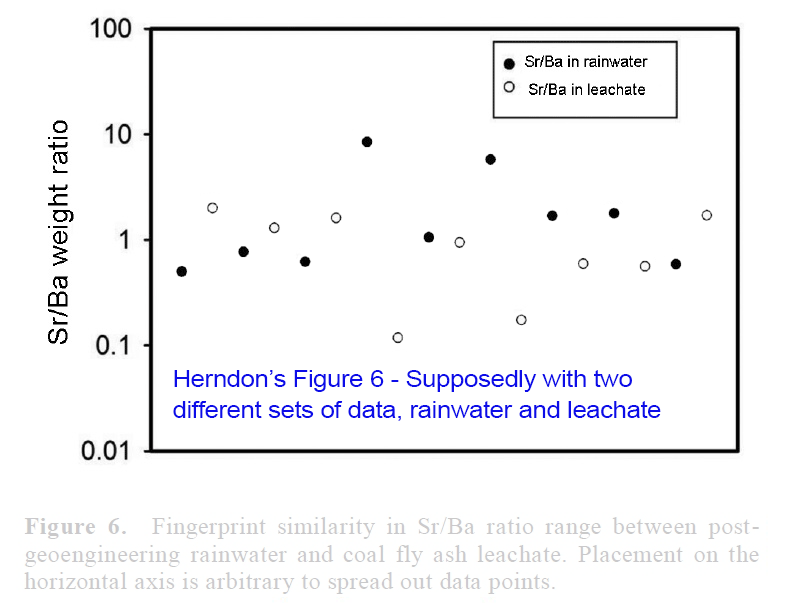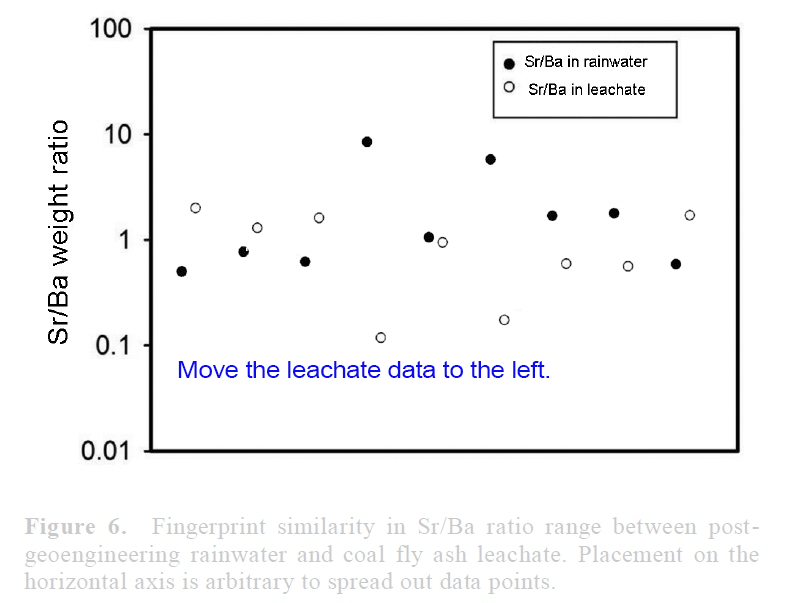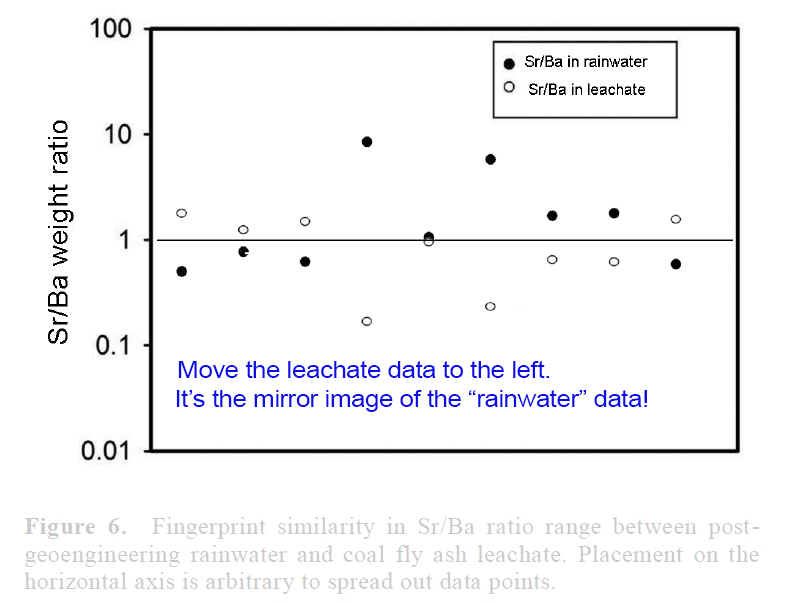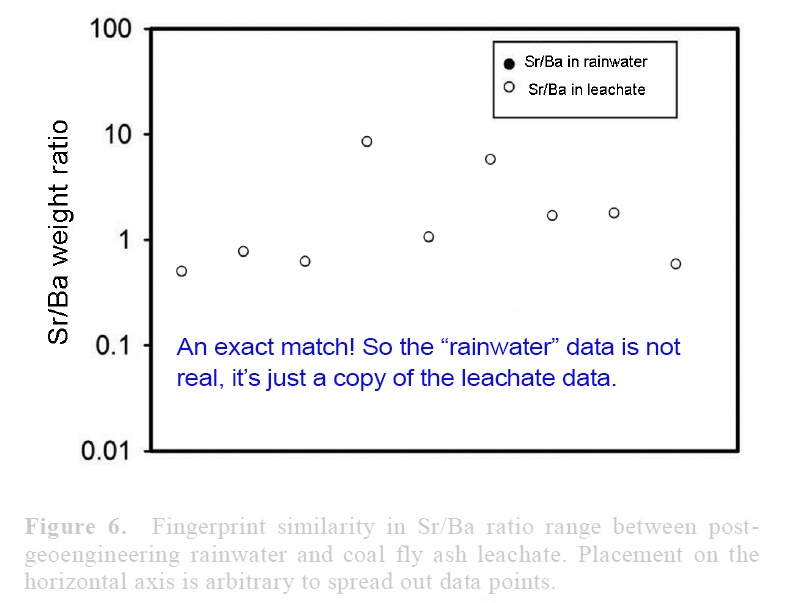
View attachment 14672
Jay Reynolds
Senior Member.
This morning I visited with Dr. Paul B. Tchounwou of Jackson State University in Jackson, Mississippi. He is Associate Dean there and Editor of the IJERPH which published Herndon's second paper. I was passing through Jackson on a trip and decided to take a half-hour to see him.
He told me that he had read the paper and was aware that it has become controversial. When asked his personal opinion of the paper he seemed hesitant to comment and did not mention anything specific. He was also aware of the publication in Current Science.
 I gave him a printout of the paper on which I had handwritten some notes about issues that have been mentioned here and at Jeffrey Beale's Blog, then I expressed my own concerns. I did give him my contact information and offered to help explain more if he desired. The meeting was brief and I thanked him for his time. He expressed that he would consider what to do very soon and thanked me for the information.
I gave him a printout of the paper on which I had handwritten some notes about issues that have been mentioned here and at Jeffrey Beale's Blog, then I expressed my own concerns. I did give him my contact information and offered to help explain more if he desired. The meeting was brief and I thanked him for his time. He expressed that he would consider what to do very soon and thanked me for the information.
He told me that he had read the paper and was aware that it has become controversial. When asked his personal opinion of the paper he seemed hesitant to comment and did not mention anything specific. He was also aware of the publication in Current Science.
Jay Reynolds
Senior Member.
One thing about the Herndon paper puzzles me. He contends that the leachate from Moreno is similar to the rainfall in respect to both containing "mobile" aluminum. This could be a real problem for him. Here are his statements from the two papers:
The leaching procedure used by Moreno, DIN 38414-S4, entails soaking the fly ash in distilled water for 24 hours while mixing, then filtering the solution using a 0.45 micron filter and then the ICP-MS element analyzer.
Herndon's rain water sample analysis is described as, "Two commercial state-of-California certified laboratories, Babcock Laboratories, Inc. and Basic Laboratory, were engaged for the San Diego rainwater analyses by inductively coupled plasma mass spectrometry. [ICP-MS]"
Typically, the chemtrail believers using Basic Laboratory in Redding, CA get results for "Total Metals" using EPA Method 200.8, which takes the rain water sample, then digests it in nitric acid, followed by ICP-MS, or takes a direct analysis of unfiltered sample followed by ICP-MS.
Essentially, EPA200.8 takes whatever is not soluble or "mobile" and dissolves it thus making it mobile.
It seems to me that Herndon cannot make the claim that what he is finding in the rainwater is "mobile" using EPA 200.8 because that test is a "Total Metals" test, NOT a soluble metals test.
This is significant because Herndon does admit that crustal aluminum is "highly immobile", yet he doesn't make any attempt to address the ordinary crustal aluminum known to be commonly found in rainwater which confounds his conclusions.
Can anyone with more detailed chemistry background provide further input?
External Quote:"The geoengineering activity via tanker-jet aircraft emplaces a non-natural, toxic substance in the Earth's atmosphere which with rainwater liberates highly mobile aluminum.".....
"Consequently, the biota of our planet, including humans, failed to develop natural defence mechanisms for exposure to chemically mobile aluminum. Globally, for the past decade or more, with dramatically increasing intensity, our planet is being deliberately and clandestinely exposed to a non-natural substance which releases toxic mobile aluminum into the environment."....................
"But to my knowledge release of mobile aluminum into the environment does not occur from natural volcanic ash."
"The Ganga Alluvial Plain, as shown in Figure 7, abuts the Himalaya Mountains, a natural barricade to the passage of clouds. Seasonally, as discovered by Jigyasu et al. 1 , rainfall delivers toxic quantities of highly mobile aluminum to the Gomati River Basin (Figure 8). I suggest that the primary source of highly mobile aluminum is aerosolized coal fly ash. This suggestion is relatively easy to verify by taking rainwater samples and analysing them for aluminum, barium and strontium"....................
https://www.metabunk.org/attachments/cs-herndon-india-2015-pdf.14598/
The problem I see is:External Quote:"The consequences on public health are profound, including exposure to a variety of toxic heavy metals, radioactive elements, and neurologically-implicated chemically mobile aluminum released by body moisture in situ after inhalation or through transdermal induction."...............
"In the 1970s acid rain [13] liberated aluminum in a chemically mobile form from otherwise inert sources, such as mine tailings, that posed an environmental health threat to a host of organisms [14,15]."......
"Although aluminum is abundant in the Earth's crust, it is highly immobile. Consequently, our planet's biota, including humans, have not developed natural defense mechanisms for exposure to chemically mobile aluminum. It is a matter of grave concern that aluminum in a chemically mobile form can be readily extracted from coal fly ash with rainwater or in situ with body fluids.".......
https://www.metabunk.org/attachments/ij-herndon-ijerp-2015-pdf.14597/
The leaching procedure used by Moreno, DIN 38414-S4, entails soaking the fly ash in distilled water for 24 hours while mixing, then filtering the solution using a 0.45 micron filter and then the ICP-MS element analyzer.
Herndon's rain water sample analysis is described as, "Two commercial state-of-California certified laboratories, Babcock Laboratories, Inc. and Basic Laboratory, were engaged for the San Diego rainwater analyses by inductively coupled plasma mass spectrometry. [ICP-MS]"
Typically, the chemtrail believers using Basic Laboratory in Redding, CA get results for "Total Metals" using EPA Method 200.8, which takes the rain water sample, then digests it in nitric acid, followed by ICP-MS, or takes a direct analysis of unfiltered sample followed by ICP-MS.
There is a difference! The Moreno results come from a true leaching test over a 24 hour agitation and filtration of solids to yield what can be considered "mobile" elements. The EPA 200.8 test takes rainwater which fell through the atmosphere for minutes, then digests the sample in acid which dissolves anything inert into a soluble "mobile" form.External Quote:For total recoverable analysis of a solid or an aqueous sample containing undissolved material, analytes are first solubilized by gentle refluxing with nitric and hydrochloric acids.
Total Recoverable Analyte - The concentration of analyte determined either by "direct analysis" of an unfiltered acid preserved drinking water sample with turbidity of <1 NTU (Section 11.2.1), or by analysis of the solution extract of a solid sample or an unfiltered aqueous sample following digestion by refluxing with hot dilute mineral acid(s) as specified in the method (Sections 11.2 and 11.3)
http://water.epa.gov/scitech/methods/cwa/bioindicators/upload/2007_07_10_methods_method_200_8.pdf
Essentially, EPA200.8 takes whatever is not soluble or "mobile" and dissolves it thus making it mobile.
It seems to me that Herndon cannot make the claim that what he is finding in the rainwater is "mobile" using EPA 200.8 because that test is a "Total Metals" test, NOT a soluble metals test.
This is significant because Herndon does admit that crustal aluminum is "highly immobile", yet he doesn't make any attempt to address the ordinary crustal aluminum known to be commonly found in rainwater which confounds his conclusions.
Can anyone with more detailed chemistry background provide further input?
Last edited:
It's unclear what he actually means by "chemically mobile aluminum". A quick search for "mobile aluminum" and "water" largely returns results about Herndon's articles.
Where "Mobile Aluminum" is actually used in the scientific literature seems to be synonymous with bioavailable aluminum, i.e. Al(III) (Al3+), the ion of aluminum that forms in acidic water. But this is a function of acidity, not aluminum (which is ubiquitous in soil).
https://books.google.com/books?id=08JkCSXX_14C&lpg=PA36&ots=1hOt4HwIwX&dq="mobile aluminum" water&pg=PA36#v=onepage&q="mobile aluminum" water&f=false

Al3+ is generally measured in very small amounts, like 1ppm (1 mg/L, or 1,000 µg/L) - because anything higher would indicate such a high acidity level that you'd have far more serious issues than aluminum toxicity.
Herndon instead seems to loosely use the term to mean very small particles of aluminum.
Where "Mobile Aluminum" is actually used in the scientific literature seems to be synonymous with bioavailable aluminum, i.e. Al(III) (Al3+), the ion of aluminum that forms in acidic water. But this is a function of acidity, not aluminum (which is ubiquitous in soil).
https://books.google.com/books?id=08JkCSXX_14C&lpg=PA36&ots=1hOt4HwIwX&dq="mobile aluminum" water&pg=PA36#v=onepage&q="mobile aluminum" water&f=false

Al3+ is generally measured in very small amounts, like 1ppm (1 mg/L, or 1,000 µg/L) - because anything higher would indicate such a high acidity level that you'd have far more serious issues than aluminum toxicity.
Herndon instead seems to loosely use the term to mean very small particles of aluminum.
Jay Reynolds
Senior Member.
Herndon has tried to tie his Indian paper into relatively high mobile aluminum found in a river in India. One problem with that is that the problem is localized and seasonal, and besides being in surface water is also in groundwater. They think that the aluminum dissolves out of mineral Biotite(black mica). If global geoengineering were taking place, the aluminum problem wouldn't be in a local groundwater or a unique river system, it would be global.
You'll find some details about it here:
http://www.researchgate.net/publica...mati_River_Basin_implications_to_human_health
You'll find some details about it here:
http://www.researchgate.net/publica...mati_River_Basin_implications_to_human_health
Attachments
Belfrey
Senior Member
I agree - when biologists talk about "mobile" or "available" aluminum, it generally refers to aluminum that is in a soluble form that will move with water and can be absorbed by plants and other living things. The solubility of both plant micronutrients and other molecules & elements in the soil (such as aluminum) will vary with pH (image source):It's unclear what he actually means by "chemically mobile aluminum". A quick search for "mobile aluminum" and "water" largely returns results about Herndon's articles.
Where "Mobile Aluminum" is actually used in the scientific literature seems to be synonymous with bioavailable aluminum, i.e. Al(III) (Al3+), the ion of aluminum that forms in acidic water. But this is a function of acidity, not aluminum (which is ubiquitous in soil).
https://books.google.com/books?id=08JkCSXX_14C&lpg=PA36&ots=1hOt4HwIwX&dq="mobile aluminum" water&pg=PA36#v=onepage&q="mobile aluminum" water&f=false

Al3+ is generally measured in very small amounts, like 1ppm (1 mg/L, or 1,000 µg/L) - because anything higher would indicate such a high acidity level that you'd have far more serious issues than aluminum toxicity.
Herndon instead seems to loosely use the term to mean very small particles of aluminum.
I agree with Jay that the two analyses are not equivalent, and that the EPA Method 200.8 will give a result for total aluminum in the sample, not just soluble aluminum.
The Jigyasu paper Herndon references in CS describes their analysis as:
https://www.metabunk.org/attachments/current-science-108-3-434-438-pdf.14719/
https://www.metabunk.org/attachments/current-science-108-3-434-438-pdf.14719/
Which appears like it would dissolve aluminum with the nitric acid before filtering. I'm not sure how they can say they are measuring dissolved aluminum when they will dissolve additional aluminum from suspended solids with their test procedure?External Quote:
All river water and groundwater samples were collected in wide mouth
250 ml polypropylene bottles (© Tarsons) with airtight
caps and were acidified in the field with HNO3 (5 ml/l).
Each sample bottle was tagged with appropriate label and
was carefully transported to the laboratory for chemical
analysis. The samples were filtered through Millipore
filtering assembly using <0.45 µm membrane filter and
analysed by Inductively Coupled Plasma-Mass Spectrophotometer
(ELAN DRC II Perkin Elmer SCIEX Instrument)
at Indian Institute of Technology Roorkee, Roorkee.
Each sample was analysed in triplicate and mean value
was taken as the result. All the samples were analysed in
the laboratory following the standard protocols9 . The
overall precision of the analytical method is about 3%.
...
9. APHA, Standard methods for the examination of water and
wastewater. American Public Health Association, Washington,
DC, 2002, 20th edn.
skephu
Senior Member.
He is not aware of that. He argues that the rainwater samples are filtered through a fine filter in the laboratory before analysis, so there cannot be any solids in there.It seems to me that Herndon cannot make the claim that what he is finding in the rainwater is "mobile" using EPA 200.8 because that test is a "Total Metals" test, NOT a soluble metals test.
Trailspotter
Senior Member.
The "identification" of "chemtrail" substances allows testing these substances for their capability of persistent trail formation. If it indeed were coal fly ash, an experimental demonstration of the persistence of a trail formed upon spraying the actual said substance in the air would be the strongest possible evidence for its use in the alleged geoengineering activity. However, so far, neither Herndon, nor his supporters have come with the (positive) results of such a simple experiment.
The "identification" of "chemtrail" substances allows testing these substances for their capability of persistent trail formation. If it indeed were coal fly ash, an experimental demonstration of the persistence of a trail formed upon spraying the actual said substance in the air would be the strongest possible evidence for its use in the alleged geoengineering activity. However, so far, neither Herndon, nor his supporters have come with the (positive) results of such a simple experiment.
And some really useful evidence would be photographs of the "ash" being sprayed from one of the planes over San Diego. Herndon could very easily take a close-up photo of one of the planes with a relatively cheap camera like the Nikon Coolpix P900.
Belfrey
Senior Member
The Jigyasu paper Herndon references in CS describes their analysis as:
https://www.metabunk.org/attachments/current-science-108-3-434-438-pdf.14719/
Which appears like it would dissolve aluminum with the nitric acid before filtering. I'm not sure how they can say they are measuring dissolved aluminum when they will dissolve additional aluminum from suspended solids with their test procedure?External Quote:
All river water and groundwater samples were collected in wide mouth
250 ml polypropylene bottles (© Tarsons) with airtight
caps and were acidified in the field with HNO3 (5 ml/l).
Each sample bottle was tagged with appropriate label and
was carefully transported to the laboratory for chemical
analysis. The samples were filtered through Millipore
filtering assembly using <0.45 µm membrane filter and
analysed by Inductively Coupled Plasma-Mass Spectrophotometer
(ELAN DRC II Perkin Elmer SCIEX Instrument)
at Indian Institute of Technology Roorkee, Roorkee.
Each sample was analysed in triplicate and mean value
was taken as the result. All the samples were analysed in
the laboratory following the standard protocols9 . The
overall precision of the analytical method is about 3%.
...
9. APHA, Standard methods for the examination of water and
wastewater. American Public Health Association, Washington,
DC, 2002, 20th edn.
This is a standard practice when sampling for total Al in surface waters, because otherwise there is often an issue with aluminum compounds adsorbing or precipitating on the wall of the container. However, it is generally not done when the goal is to determine the amount of dissolved aluminum, because an acid preservative will change solubility.
As explained in Determination of Dissolved Aluminum in Water Samples (1982):
The EPA procedure for Method 3005A, Acid Digestion of Waters for Total Recoverable or Dissolved Metals for Analysis by FLAA or ICP Spectroscopy, clearly states that filtering should be done before acidification: "When analyzing for total dissolved metals filter the sample, at the time of collection, prior to acidification with nitric acid."External Quote:Another problem encountered by several workers in the determination of Al and other trace metal is absorption of the dissolved elements on the sampling container wall (Stoffyn, 1979). Acidification of the sample with Ultrexi/ nitric acid to pH <1.5 was found to eliminate this problem (Subramanian and others, 1978). However, acidification changes distribution of elemental species, which is not desirable, particularly in Al determination. Colloidal polymeric aluminum and strong alumino-organic complexes are acid soluble. Stoffyn (1979), p. 121-149, used Teflon bottles to reduce the absorption of dissolved Al on the sampling container walls. Barnes (1975), suggested extraction of Al immediately after collection if only dissolved equilibrium Al species are to be determined.
Mark Barrington
Active Member
A decent tripod doesn't cost a lot. A monopod even less. I'm assuming the coolpix has a tripod mount, most cameras do.I have a similar Nikon Coolpix, I wish I could hold it that steady.
deirdre
Senior Member
you don;t.But how do you move it around to follow the plane when it's on a tripod? You can see I'm a total moron when it comes to cameras.
Mark Barrington
Active Member
Most tripods allow you to pan along one axis while the other axes are locked, but it's hard to move smoothly unless you have a more expensive tripod with some damping. you can either be quick, as Dierdre says, or use something like a monopod which steadies the camera but doesn't keep you from moving it.But how do you move it around to follow the plane when it's on a tripod? You can see I'm a total moron when it comes to cameras.
JFDee
Senior Member.
Today, something has appeared which comes close to an official statement from MDPI:
http://scholarlyoa.com/2015/08/25/m...-swiss-chinese-publisher-mdpi/#comment-343756
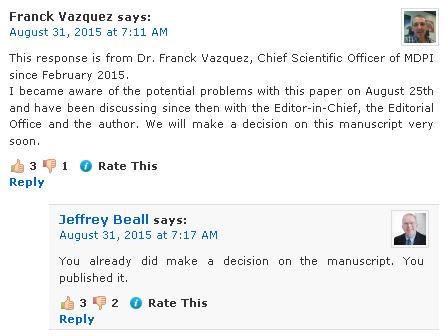
http://scholarlyoa.com/2015/08/25/m...-swiss-chinese-publisher-mdpi/#comment-343756
Jay Reynolds
Senior Member.
This note was recently attached to Herndon's paper:
External Quote:† Note added by the Publisher: This paper attracts great attention and might be controversial. We are currently re-evaluating the paper, re-assessing the comments made by the three reviewers. Please take the conclusions of this paper with care until the re-evaluation is complete.
http://www.mdpi.com/1660-4601/12/8/9375
deirdre
Senior Member
i see Micks infographic got 22 thumbs up over there.. he only got 5 here. Guess he's hanging with the wrong crewToday, something has appeared which comes close to an official statement from MDPI:
http://scholarlyoa.com/2015/08/25/m...-swiss-chinese-publisher-mdpi/#comment-343756
On Aug 25th a new version of the paper was uploaded with the following text removed:
There seems to be no other changes, although the PDF was saved with different settings/software, so is considerably smaller (presumably the images are more compressed)External Quote:I thank Weidan Zhou for professional statistics advice.
Attachments
skephu
Senior Member.
I can't see this note there.This note was recently attached to Herndon's paper:
External Quote:† Note added by the Publisher: This paper attracts great attention and might be controversial. We are currently re-evaluating the paper, re-assessing the comments made by the three reviewers. Please take the conclusions of this paper with care until the re-evaluation is complete.
http://www.mdpi.com/1660-4601/12/8/9375
Psychic
Senior Member
No one likes retractions, so I guess they're dragging their feet. But I've never seen a journal distance itself from a paper because of 'controversy' alone, so it looks like they're already convinced it's probably bunk.This note was recently attached to Herndon's paper:
External Quote:† Note added by the Publisher: This paper attracts great attention and might be controversial. We are currently re-evaluating the paper, re-assessing the comments made by the three reviewers. Please take the conclusions of this paper with care until the re-evaluation is complete.
http://www.mdpi.com/1660-4601/12/8/9375
That said, if they do eventually retract it, that could turn many chemmies against the scientific establishment itself, so it may be politically pragmatic to avoid that.
That said, if they do eventually retract it, that could turn many chemmies against the scientific establishment itself, so it may be politically pragmatic to avoid that.
I would think that's not a major concern of the Journal. They want to try to salvage their reputation somewhat.
Of course it will be viewed as "censorship", because the chemtrail believers will continue to think the paper is correct (largely without actually reading it, or the objections listed here). Ultimately though it's best not to have such obvious bunk in scientific journals (even if the journals don't have a good reputation).
It's hidden in the "Author affiliation" section.I can't see this note there.

JFDee
Senior Member.
You could take this in a totally different way:i see Micks infographic got 22 thumbs up over there.
It was your discovery that has shaken and excited the academic world ...
Last edited:
deirdre
Senior Member
Ray Von Geezer
Senior Member
The paper at MDPI has been retracted - MDPI Retraction.
 And Jay for bringing it to their attention.
And Jay for bringing it to their attention.
Ray Von
Many (all?) of the errors seem to have been those identified here so well done all you boffinsExternal Quote:It was brought to my attention that there are problems related to the recently published article "Evidence of Coal-Fly-Ash Toxic Chemical Geoengineering in the Troposphere: Consequences for Public Health" [1].
Together with the Chief Scientific Officer, Dr. Franck Vazquez, and the Editorial office, we re-evaluated the paper, re-assessed the comments made by the three reviewers and note the following crucial concerns:
• The value for average leachate concentration of Aluminum mentioned in Table 1 and used by the author to normalize the data presented in Figures 2, 3, 4 and 5 is incorrect. The author uses 70,000 μg/kg, while the correct value resulting from the un-leached European coal fly ash samples measurements published by Moreno et al. [2]) is 140,000,000 μg/kg. This error invalidates the conclusions of the article.
• The chemical compositions obtained for rainwater and HEPA air filter dust are only compared to chemical compositions obtained for coal-fly-ash leaching experiments [2]. The author did not attempt to compare his results to chemical compositions of other potential sources. Thus, at this stage, the work is preliminary since it is not clear what the source of these chemicals is.
• The language of the paper is often not sufficiently scientifically objective for a research article.
Consequently, we have decided to retract the article. This paper is thus declared retracted and shall be marked accordingly for the scientific record.
MDPI takes the responsibility to enforce strict ethical policies and standards very seriously. We aim to ensure the publication of only truly original and scientific works. MDPI would like to apologize to the readers of IJERPH that this article was published with the errors mentioned above.
We sincerely appreciate the efforts of those who bring aspects of scientific error to our attention in an effort to maintain scientific integrity.
Ray Von
NoParty
Senior Member.
Absolutely. To hard-core "chemtrail" believers:...the chemtrail believers will continue to think the paper is correct (largely without actually reading it, or the objections listed here).
A) MDPI publishing Herndon's Coal-Fly-Ash paper = "We are proved right!" and
B) MDPI retracting Herndon's Coal-Fly-Ash paper = "We are proved right!"
"All roads lead to our belief!" But for the marginal folks, this could be significant:
The big "proof" chemtrailers have been saying makes their case, shown to be bad science
Trailspotter
Senior Member.
To hard-core "chemtrail" believers:
A) MDPI publishing Herndon's Coal-Fly-Ash paper = "We are proved right!" and
B) MDPI retracting Herndon's Coal-Fly-Ash paper = "We are proved right!"
No Party for the Metabunk
skephu
Senior Member.
Hmmm, it seems to me there are some errors in the retraction, too 
This was not the leachate concentration but the unleached one, and it wasn't used (presumably) for all the figures mentioned, only Figures 4 and 5.External Quote:The value for average leachate concentration of Aluminum mentioned in Table 1 and used by the author to normalize the data presented in Figures 2, 3, 4 and 5 is incorrect. The author uses 70,000 μg/kg, while the correct value resulting from the un-leached European coal fly ash samples measurements published by Moreno et al. [2]) is 140,000,000 μg/kg.
MikeG
Senior Member.
Absolutely. To hard-core "chemtrail" believers:
A) MDPI publishing Herndon's Coal-Fly-Ash paper = "We are proved right!" and
B) MDPI retracting Herndon's Coal-Fly-Ash paper = "We are proved right!"
"All roads lead to our belief!" But for the marginal folks, this could be significant:
The big "proof" chemtrailers have been saying makes their case, shown to be bad science
Agreed. The true believers will blame the retraction on the machinations of the "global power structure" and their minions.
As for other people on the fence or still seeking answers, perhaps the retraction will spur some important doubts about chemtrail "science" and how it should stack up against peer-reviewed research.
We'll see.
Hama Neggs
Senior Member
Most people will never know about the retraction, since they will just be looking at cherry-picked bit of it published on conspiracy sites.
Similar threads
- Replies
- 45
- Views
- 5K
- Replies
- 21
- Views
- 3K
- Replies
- 129
- Views
- 22K
- Replies
- 3
- Views
- 4K
- Replies
- 34
- Views
- 14K