Woody
Member
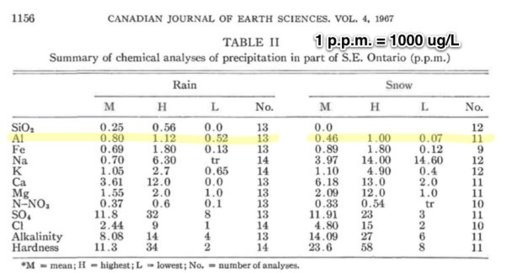
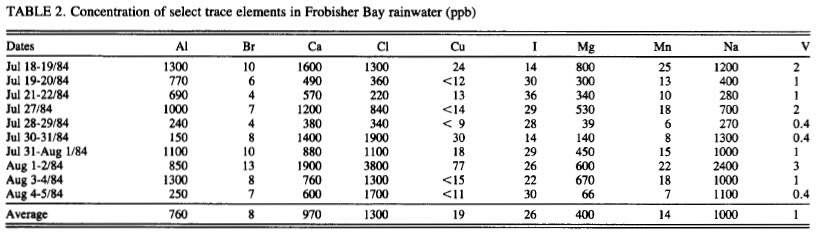
Because a nut believed in chemtrails doesn't prove they do not exist, and because a nut said there was WMD's in Iraq doesn't mean they had them either? I have been watching these posts on chemtrails, I have actually seen the chemical tanks on the planes with binoculars and chemtrails and contrails are in the sky together at the same time on many days. Water samples spike in Magnesium 50x the normal levels mid continent (Magnesium lowers the further you move away from the oceans). I was one of the few said it was a conspiracy when we went to war over WMD's, and I was right then too. They are chemicals and conspiracy theorists are taking off with this due to the lack of transparency. It's being done to cool the planet duplicating the effect of air pollution without the side effects, they hope. Its not mind control or anything like that. And by the way, its working, came home to 2 feet of snow, soon we can sun bathe on the ice out on the lakes.