Belfrey
Senior Member
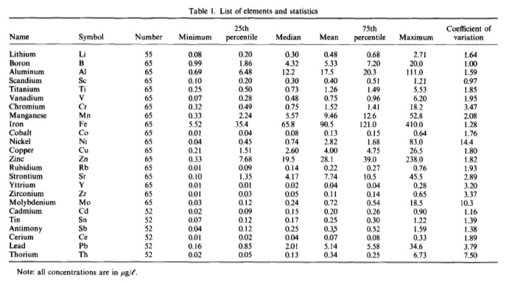
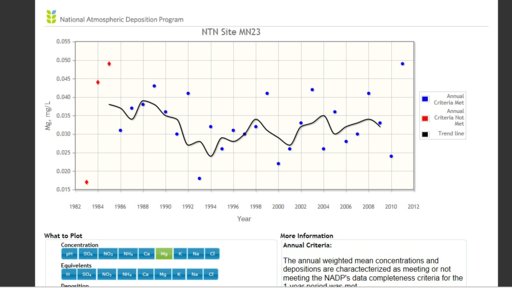
Depends, magnesium is not regulated and is found in the Ocean in heavy concentrations so it is common to have high magnesium levels near the oceans. High magnesium levels inland come after the rain has fallen and is picked up through the soils. The farmers have researched the impacts on their soils so they measure samples for irrigation of water and water for their livestock. Magnesium Oxide, if it is this, is unregulated and OSHA did attempt to regulate it due to the impact on workers and towns near processing plants. Here is a portion of that report "The local population may be exposed to MgO through inhalation of contaminated air released into the atmosphere from magnesium producing facilities. The possibility that such exposures may cause adverse effects in residents of cities located near magnesite-processing facilities was examined, with studies showing pregnancy complications, increased morbidity from respiratory and digestive tract diseases in children, and magnesium excretion and alkaline urine in children cited as evidence to support an association (Reichrtová & Taká…, 1992b)." As far as regulation, "OSHA's January 19, 1989 Final Rule on Air Contaminants contained an 8-hour timeweighted average (TWA) PEL of 10 mg/m3 (total particulate) and 5 mg/m3 (respirable particulate) for magnesium oxide fume. NIOSH did not concur with OSHA's limit for magnesium oxide fume, noting that exposure to magnesium oxide may cause chronic respiratory disease in addition to metal fume fever. The 1989 rule was remanded by the US Circuit Court of Appeals and the limits are not currently in force (NIOSH, 2001)." USDA probably issues the 150 ug/L content for farms
"Probably", or does it? Where did you get the figure of 150 ug/L in rainwater, and can you find any agency which lists that as a regulatory limit? Your paragraph refers to particulate emissions of MgO from industrial sources.