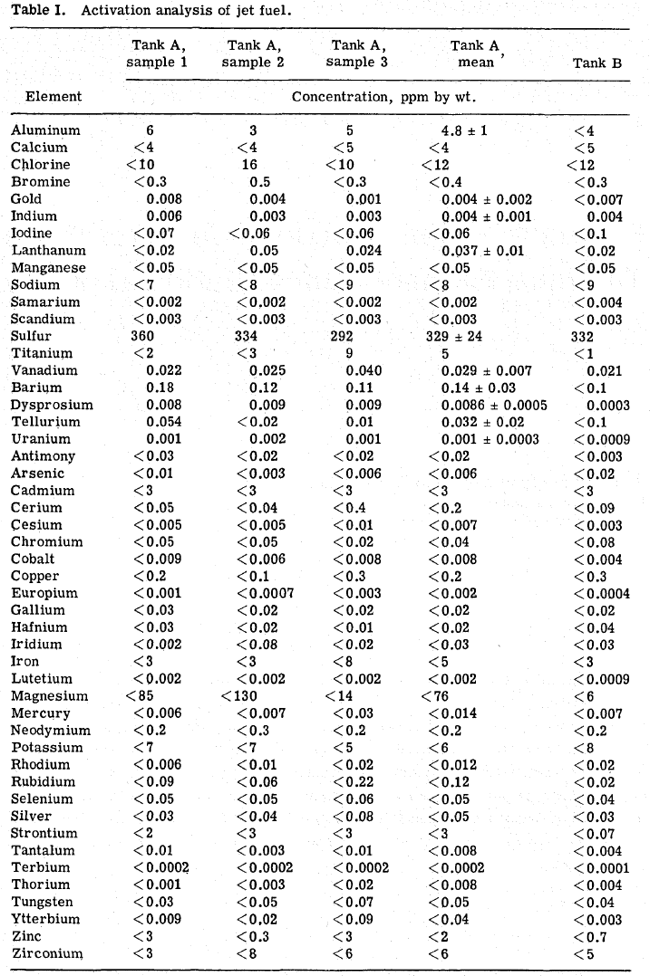
2.0 CHFMTCAL GETTERS
Chemical methods were the first approach evaluated during this
program. Among the chemical methods, "getters", metals or alloys
with chemically active surfaces, appeared attractive be cause they
react with oxygen to form insoluble oxides. They have been widely
used in the semiconductor and nuclear fuels industries. Many of
these are transition metals which were deemed unacceptable for this
application because of the potential for catalyzing reactions in
fuel. Barium metal, however, is a commonly used getter and looked
attractive. Barium reacts readily with oxygen and water, but the
reaction is not as exothermic as the reaction of other metals such
as sodium. The relevant reactions of barium are:
2Ba + 02 - 2BaO
Ba + H20 - BaO + H2
BaO + H20 - Ba(OH)2
Laboratory experiments were performed to determine the
effectiveness of this method. The initial experiments were
conducted with JP-5 in a threeneck round bottom flask. Two of the
necks contained stopcocks attached to ground glass joints. One
stopcock was used to admit nitrogen gas while the other was used
to vent the flask. The third neck held the probe for a Yellow
Springs Instrument International, Inc. model 58 dissolved oxygen
meter. One liter of JP-5 fuel was used. The barium metal was
purchased in 6 mm diameter sticks (granulated barium metal is not
currently available from laboratory suppliers). These sticks were
cut into pieces approximately 6 mm in length. The barium was
stored in oil under a nitrogen atmosphere in an attempt to prevent
its surface from oxidizing. The JP-5 fuel was saturated with air
prior to the experiment by bubbling air through it.
In the first experiment, fuel and barium were stirred together
in the flask under a nitrogen atmosphere. After 30 minutes, the
oxygen concentration had decreased to 50% of the initial concentration.
It was observed, however, that this result could be obtained
simply by stirring the fuel under a nitrogen atmosphere without the
barium metal. The presence of white solid did indicate that some
barium had reacted. Subsequent experiments were performed with air
initially present and no nitrogen purging of the flask.
In order to increase the exposed surface area of barium, the
threenecked flask was replaced with a blender. The lid was sealed
to the container to prevent air leakage after the deoxygenation
process began. The system was simplified by using cyclohexane
rather than JP-5. The cyclohexane was mixed with the pieces of
barium in the blender until the barium was broken into very small
particles. After 30 minutes, the oxygen concentration was 4% of
the initial concentration. The absolute concentration of oxygen
in the cyclohexane could not be determined by our measuring
instrumentation which gives a relative indication, however,
assuming the air saturated concentration to be 50 ppm then 4% would
be 2 ppm. This value may well reflect the air-tightness of the
blender rather than the limit of the deoxygenation capability of
the barium. A white solid was observed in the liquid. At the
conclusion of the experiment, solid barium metal remained in the
container. The white solid was filtered from the liquid with
Whatman No. 2V filter paper. This paper retains solids larger than
8 microns in size. The filtered solution was observed to be clear
and did not scatter light, thus indicating that all solid
by-products had been removed by the filter. It was also observed
that a coating of white solid remained on the walls of the
container.
Calculations of the quantity of barium metal required to
stoichiometrically react with 10,000 gallons of fuel with 50 ppm
oxygen yield a value of 35 lbs of barium. Additionally, twenty-two
lbs of barium react with 70 ppm of water in 10,000 gallons of fuel.
The actual quantity of barium metal required to react with both the
oxygen and water is less than the total of these two values because
barium oxide itself reacts with water. Barium sells commercially
for approximately $20/lb which is in the range of economically
feasibility. However, barium is costly enough that recycling
technique should be explored for large throughput operations.