You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Investigating "Active Thermitic Material Discovered in Dust from the 9/11 WTC Catastrophe"
- Thread starter Mick West
- Start date
-
- Tags
- ae911truth nanothermite
Mendel
Senior Member.
I looked through the wikipedia page on kaolin, all of the processes to make kaolin chemically are way more complex than "just burn some stuff", and typically involve controlled hydrothermal conditions as well as specific precursor molecules.Can you show me that silicone and Al do not produce kaolin when ashed?
John J.
Senior Member.
No-one should be abusing anyone here. But sometimes people get frustrated, and we all make slips or post a bit rashly.all I'm getting is abuse and contradictions.
I think you're getting contradictions because (I would guess) the majority of people here, including many who have made an effort to appraise themselves of the facts, simply don't accept what appears to be your underlying thesis- that the WTC might have been demolished using pre-placed munitions or incendiaries of some description.
A suitably resourced chemist, with unlimited access to scavenge at the WTC site with its grim wreckage of iron, steel, aluminium, many other metals and many plastics and other hydrocarbons that escaped combustion might well have been able to construct a viable explosive device or thermite analogue. But that wouldn't be evidence that any such device (or the necessary compounds/ mix of materials it requires) ever existed. Equally, it would be nigh-on impossible to prove that it had not existed.
But for most people, it has been convincingly demonstrated that what many of us intuitively believed at the time was correct- an airliner laden with fuel was crashed into each tower, and that was a patently sufficient cause of the tower's collapse.
It's worth noting that the small minority of people with any credibility at all (i.e. not political extremists or apocalyptically-minded conspiracy theorists) who claim to take the "planned sabotage" theory seriously, e.g. Steven E. Jones, carry on with their lives perfectly normally.
Despite publicly, and repeatedly, implying that an evil cabal within the United States organised the mass murder of 2,977 innocent people (and presumably 19 al-Qaeda "members") people like Jones live in the open, pay their taxes, go shopping etc. and are happy to discuss their ideas in public forums without any apparent concern for their own safety.
Their actions are at variance with their claims.
Many people here are happy to discuss all sorts of issues, but there's no guarantee or obligation for them to agree with any stated viewpoint. Most of us (I think) want to find out things and establish facts about subjects that interest us on this forum, but I guess we all have to accept that we might not get definitive answers, that are convincing to us as individuals, about some subjects by their very nature.While 9 out of 10 voices tell me not too… there is that one that I spend most my day trying to talk out of domestic terror to try to lite a fire under this issue. I want answers and am getting desperate.
Sometimes it's helpful to embrace- or at least provisionally accept- uncertainty, while remaining open to new information (and sometimes other points of view).
It seems to me most posters here are broadly friendly (or at least civil most of the time!) even if we have occasional spats.
Must seem like herding cats to Mick, Landru, flarkey et al.
But no-one here can be responsible for your beliefs or choices. Generally though, it's unwise to make serious decisions out of frustration or based on suspicions that are widely held to be incorrect by the majority of essentially decent and bright people.
Maybe review your post- no-one would want you to get into trouble unnecessarily (and VPNs etc. aren't always a panacea).
As I said, most of us sometimes get frustrated, or perhaps post in haste.
Oystein
Senior Member
@Kitcosby, I asked you twice, and bolded the question to encourage you to really really answer straight and honestly and prominently - but you chose not to. So I am asking again:
Please acknowldege prominently that you have been informed that the red layers of the chips are SEVERAL DIFFERENT MATERIALS!
---------------------------
Now on to responding to your post:
Also I didn't say that that epoxy released that many times as much heat as "thermite", I said the paint released that many times more heat than the TINY proportion of thermite that could at most be in it would release if we imagine, if we fantasize that any of the Al were elemental and did participate in a thermite reaction.
So, first thing for you, you need to be clear you understand basic physics terms, that you don't confuse "power" with "heat", that you don't conflate "thermite" with "thermitic material". And to read carefully what I actually write.
We MUST pick our data carefully once we have understood that the red layers are SEVERAL DIFFERENT MATERIALS. You cannot conflate observations made on several different materials and then pretend you can use that data without "picking", i.e. without making sure you know which of the specimens are the same material, and which are different ones.
How did you arrive at making such distinctions?
How did you arrive at that "90%" figure? Please show your work!
How did you determine the "'silicone' and epoxy blend" would "provide most of [the red substance's] energy"? By "most" you mean ">50%", right?
So you agree that only a minority of the energy (heat) released when the chips burn comes from thermite?
Do you understand that this result immediately means that the DSC curves in Figure 19 of Harrit et al - their general shapes, their peak location and height - are mostly determined by this smoldering of the binder, and NOT by any hypothetical thermite reaction?
There is no Al-only phase. There is no type of particle anywhere, in any specimen, neither in Harrit et al nor in Millette, nor in any of the apocryphal data provided by Mark Basile, Kevin Ryan, or Harrit, Jones, Jeff Farrer elsewhere, that shows any constituent substance of any red layer specimen that is shown to be Al only.
You may object that Figure 17 of Harrit et al shows "mostly Al", but that single data point is problematic:
a) This is the only XEDS they show anywhere that was won from a 10 kV only electron beam - no explanation if given for this.
b) The graph is essentially zero beyond the tiny Si-peak, which makes me suspect it was done with an even lower beam energy, or it could have been manipulated (I know they HAVE manipulated XEDS graphs, to excluse the labels of some elements that are clearly there but perhaps deemed "inconvenient" to them)
c) Jones failed to indicate where, exactly, on the chip he found this spot - the caption merely says "region of high aluminum concentration on the MEK-soaked red chip" - I'll explain below, why this is a huge problem:
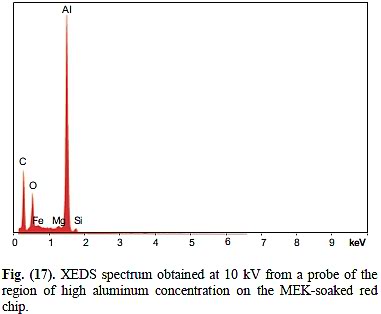
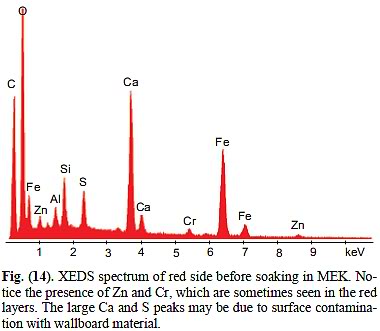
d) It is customary in electron microscopy to accompany a quantitative reading, such as an XEDS graph, from a spot with an electron microscopy image of the spot and the region of the spot, so you can see what they were focusing on - Jones failed to show us ANY e-microscopic detail of the MEK-soaked chip. So, did he focus on one of the hexagonal platelets? -> Problem is, we don't even know at all that this particular chips contained any such platelets to begin with. Problem is, as per Figure 14, wich is the XEDS spectrum before MEK-soaking, we know that chip contained significant Zn, Mg, S and Ca on top of the five elements we have established for the four specimens in Figures 5 through 11 (C,O, Al, Si, Fe). And uhm yes, there IS Mg (magnesium) in this spectrum: It's the smaller peak between Zn and Al at 1.25 keV. There can be no serious doubt that Jones edited it out. True, Jones suspects, plausibly, that the surface of this chip was contaminated with fine gypsum dust, and that this could explain the presence of sulfur (S) and (some!) of the calcium (Ca) - but he has no explanation for the Zn and the Cr. Nor for the Mg. Nor for all of the Ca (there is far more Ca relative to S implied by the graph than could be explained by gypsum only), nor for the fact that there is roughly twice as much Si as Al. And so we must conclude at this point that this is a material DIFFERENT from the one that contains platelets, and he was WRONG to assume that it would have contained Al and Si together, as it was in the four chips of Figures 5-11.
And now the explanation why not showing the exact spot that Jones found mostly only only Al is problematic:
The XEDS map, Figure 15, was done "at a beam energy of 10 kV" (page 17).
An "XEDS map" is essentially your electron microscope in autopilot mode, systematically scanning your specimen and doing brief XEDS spectra for each "pixel", and analyzing automatically wether any given spot contains chosen elements above some threshold value of x-ray counts. Because the process of doing XEDS takes a little time, and because an XEDS map contains hundreds, perhaps thousands, or tens of thousands even, spots of "pixels", and an XEDS measurement is taken on each one, to save time, energy, data volume, each spot yields only a limited number of returned and counted x-rays.
Now, I suspect strongly that the XEDS in Figure 17, with the high Al peak, is simply one of the thousands of individual spots in the XEDS map, Fig 15. Reasons: i.) 10 keV electron beam ii.) very limited x-ray count (there is almost no backgrund noise, meaning there is a low x-ray count overall). But look at the x-ray map - and the regions that are high in Al, but NOT high in any other elements:
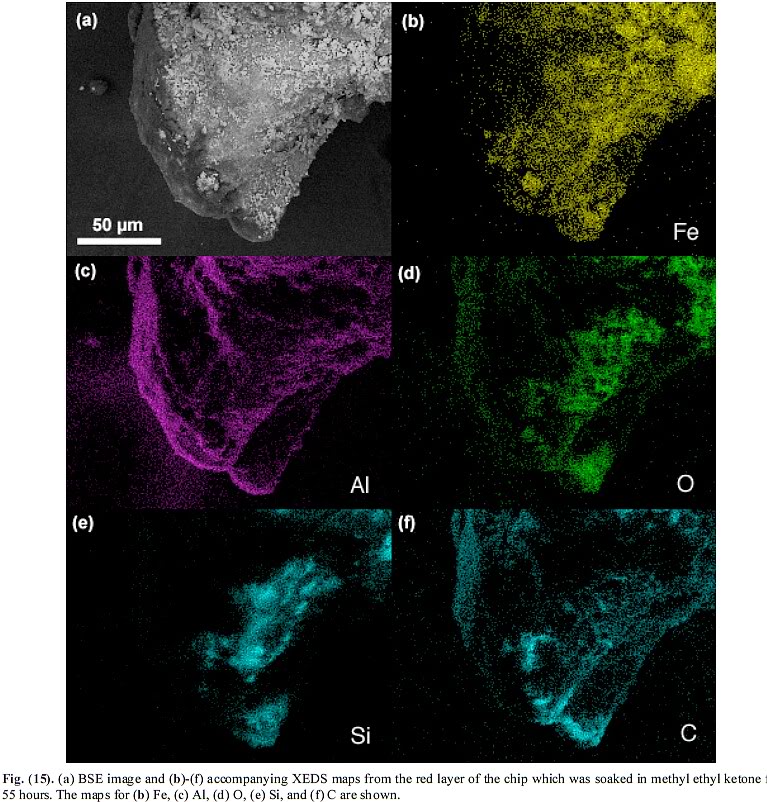
Do you see how there is a region to the left of the specimen, i.e. outside the specimen, i.e. NOT on the specimen at all and rather associated with the sample holder, where the XEDS map yields many spots with significant Al, a moderate number of spots with some O, but almost no spots with Si, C or Fe?
This means that the XEDS dataset represented by Figure 15 MUST include some spots that show plenty of Al, some O and little of everything else, that are NOT ON THE CHIP AT ALL!!!
In other words: Jones found some elemental Al, or Al-oxide, or both - on the sample holder, not in the specimen!
And so, other than this highly dubios data point of Figure 17, there is ZERO evidence anywhere for elemental Al.
If you want to claim that presence of kaolin after ashing is not evidence of the presence of kaolin before ashing, then you necessarily imply that kaolin can be formed during the ashing process.
You would have to provide an argument that supports your claim.
You might increase that confidence some if you would acknowldege prominently that you have been informed that the red layers of the chips are SEVERAL DIFFERENT MATERIALS!
This is a really really important fact to know and understand when discussing the red/gray chips. As long as we don't know with certainty that you will always always always keep this important fact in mind when discussing the red/gray chips, continuing the discussion with you is almost useless.
Please acknowldege prominently that you have been informed that the red layers of the chips are SEVERAL DIFFERENT MATERIALS!
---------------------------
Now on to responding to your post:
Uhm - I didn't say "more powerful", I said "released more heat" by such factors.The smoldering of epoxy between 37-46 more powerful then thermite is not an unusual exothermic property?
Also I didn't say that that epoxy released that many times as much heat as "thermite", I said the paint released that many times more heat than the TINY proportion of thermite that could at most be in it would release if we imagine, if we fantasize that any of the Al were elemental and did participate in a thermite reaction.
So, first thing for you, you need to be clear you understand basic physics terms, that you don't confuse "power" with "heat", that you don't conflate "thermite" with "thermitic material". And to read carefully what I actually write.
All of the samples with those hexagonal platelets have Al=Si.The samples tested all had varying ratios of Al to Si, with Si found 3x the Al in one sample.
No.To say they were approx equal is cherry picking data.
We MUST pick our data carefully once we have understood that the red layers are SEVERAL DIFFERENT MATERIALS. You cannot conflate observations made on several different materials and then pretend you can use that data without "picking", i.e. without making sure you know which of the specimens are the same material, and which are different ones.
??I am frustrated with the confabulation between the mellue
Can you show the images where you see "several paints", and the images where you see "traces of the original primer", and the images where you see "a substance that appears to be two paints painted together", so we know what you are talking about?I do see several paints, traces of the original primer, and a substance that appears to be two paints painted together.
How did you arrive at making such distinctions?
Which 'silicone'? I see you put this word, and only this word, in single quote marks. Is this because even you recognize that there is ZERO evidence for 'silicone'?The 'silicone' and epoxy blend make up 90% of the red substance and provide most of it's energy.
How did you arrive at that "90%" figure? Please show your work!
How did you determine the "'silicone' and epoxy blend" would "provide most of [the red substance's] energy"? By "most" you mean ">50%", right?
So you agree that only a minority of the energy (heat) released when the chips burn comes from thermite?
Do you understand that this result immediately means that the DSC curves in Figure 19 of Harrit et al - their general shapes, their peak location and height - are mostly determined by this smoldering of the binder, and NOT by any hypothetical thermite reaction?
In fact, you imply that it is essentially a plastic explosive and NOT "thermite"Thus it is more similar to a plastic explosive then conventional thermite.
That what was applied in two layers?Now I would love to see evidence that it was applied in two layers as that would render the feared reaction harmless.
Are you claiming that kaolin formed during the process of ashing the specimen?All the tests that found kaolin were on ashed samples.
Well, uhm, yeah, when you have experimental data and you interpret it expertly, you infer. That's science.The rest of the evidence against elemental Al is inferred.
There is no Al-only phase. There is no type of particle anywhere, in any specimen, neither in Harrit et al nor in Millette, nor in any of the apocryphal data provided by Mark Basile, Kevin Ryan, or Harrit, Jones, Jeff Farrer elsewhere, that shows any constituent substance of any red layer specimen that is shown to be Al only.
You may object that Figure 17 of Harrit et al shows "mostly Al", but that single data point is problematic:
a) This is the only XEDS they show anywhere that was won from a 10 kV only electron beam - no explanation if given for this.
b) The graph is essentially zero beyond the tiny Si-peak, which makes me suspect it was done with an even lower beam energy, or it could have been manipulated (I know they HAVE manipulated XEDS graphs, to excluse the labels of some elements that are clearly there but perhaps deemed "inconvenient" to them)
c) Jones failed to indicate where, exactly, on the chip he found this spot - the caption merely says "region of high aluminum concentration on the MEK-soaked red chip" - I'll explain below, why this is a huge problem:
d) It is customary in electron microscopy to accompany a quantitative reading, such as an XEDS graph, from a spot with an electron microscopy image of the spot and the region of the spot, so you can see what they were focusing on - Jones failed to show us ANY e-microscopic detail of the MEK-soaked chip. So, did he focus on one of the hexagonal platelets? -> Problem is, we don't even know at all that this particular chips contained any such platelets to begin with. Problem is, as per Figure 14, wich is the XEDS spectrum before MEK-soaking, we know that chip contained significant Zn, Mg, S and Ca on top of the five elements we have established for the four specimens in Figures 5 through 11 (C,O, Al, Si, Fe). And uhm yes, there IS Mg (magnesium) in this spectrum: It's the smaller peak between Zn and Al at 1.25 keV. There can be no serious doubt that Jones edited it out. True, Jones suspects, plausibly, that the surface of this chip was contaminated with fine gypsum dust, and that this could explain the presence of sulfur (S) and (some!) of the calcium (Ca) - but he has no explanation for the Zn and the Cr. Nor for the Mg. Nor for all of the Ca (there is far more Ca relative to S implied by the graph than could be explained by gypsum only), nor for the fact that there is roughly twice as much Si as Al. And so we must conclude at this point that this is a material DIFFERENT from the one that contains platelets, and he was WRONG to assume that it would have contained Al and Si together, as it was in the four chips of Figures 5-11.
And now the explanation why not showing the exact spot that Jones found mostly only only Al is problematic:
The XEDS map, Figure 15, was done "at a beam energy of 10 kV" (page 17).
An "XEDS map" is essentially your electron microscope in autopilot mode, systematically scanning your specimen and doing brief XEDS spectra for each "pixel", and analyzing automatically wether any given spot contains chosen elements above some threshold value of x-ray counts. Because the process of doing XEDS takes a little time, and because an XEDS map contains hundreds, perhaps thousands, or tens of thousands even, spots of "pixels", and an XEDS measurement is taken on each one, to save time, energy, data volume, each spot yields only a limited number of returned and counted x-rays.
Now, I suspect strongly that the XEDS in Figure 17, with the high Al peak, is simply one of the thousands of individual spots in the XEDS map, Fig 15. Reasons: i.) 10 keV electron beam ii.) very limited x-ray count (there is almost no backgrund noise, meaning there is a low x-ray count overall). But look at the x-ray map - and the regions that are high in Al, but NOT high in any other elements:
Do you see how there is a region to the left of the specimen, i.e. outside the specimen, i.e. NOT on the specimen at all and rather associated with the sample holder, where the XEDS map yields many spots with significant Al, a moderate number of spots with some O, but almost no spots with Si, C or Fe?
This means that the XEDS dataset represented by Figure 15 MUST include some spots that show plenty of Al, some O and little of everything else, that are NOT ON THE CHIP AT ALL!!!
In other words: Jones found some elemental Al, or Al-oxide, or both - on the sample holder, not in the specimen!
And so, other than this highly dubios data point of Figure 17, there is ZERO evidence anywhere for elemental Al.
That's a bad attempt at trying to reverse the burden of evidence.Can you show me that silicone and Al do not produce kaolin when ashed?
If you want to claim that presence of kaolin after ashing is not evidence of the presence of kaolin before ashing, then you necessarily imply that kaolin can be formed during the ashing process.
You would have to provide an argument that supports your claim.
You are not inspiring confidence that you are discussing here in good faith.Tongue in cheek! I am insane.
While 9 out of 10 voices tell me not too… there is that one that I spend most my day trying to talk out of domestic terror to try to lite a fire under this issue. I want answers and am getting desperate.
You might increase that confidence some if you would acknowldege prominently that you have been informed that the red layers of the chips are SEVERAL DIFFERENT MATERIALS!
This is a really really important fact to know and understand when discussing the red/gray chips. As long as we don't know with certainty that you will always always always keep this important fact in mind when discussing the red/gray chips, continuing the discussion with you is almost useless.
NorCal Dave
Senior Member.
Do you see how there is a region to the left of the specimen, i.e. outside the specimen, i.e. NOT on the specimen at all and rather associated with the sample holder, where the XEDS map yields many spots with significant Al, a moderate number of spots with some O, but almost no spots with Si, C or Fe?
Just for some of us non-chemists following along, I take it to mean these points in the sample that are not the sample, but the base or sample holder as you call it:
Oystein
Senior Member
Yes.Just for some of us non-chemists following along, I take it to mean these points in the sample that are not the sample, but the base or sample holder as you call it:
Similar threads
- Replies
- 129
- Views
- 22K
- Replies
- 34
- Views
- 14K
- Replies
- 36
- Views
- 12K
- Replies
- 0
- Views
- 4K
