The experience debunking the angle cut column was interesting, as it showed that even though something may well have been debunked sufficiently for most people years ago, that debunking information is often inaccessible and arguable - sometimes buried in discussion threads. The cut column discussion resulted in an inarguable debunk, with accompanying simple graphics.

So I'd like to do the same with one of the most popular claims - that thermite was found in the remains of the towers. Specifically with this 2009 paper:
http://www.911research.wtc7.net/mirrors/bentham_open/ActiveThermitic_Harrit_Bentham2009.pdf
I recognize this is old ground, however my goal here is to produce a go-to debunk of the paper using past, synthesizing past debunks with any more recent information, and presenting it in an accessible manner.
So let's begin by gathering the past debunks, much of which was done deep within the JREF/ISF forum.
Oy's summary likely refers to these thread on ISF (formally JREF)
So what I'd like to do is create a simple yet deep summary of the evidence for paint, and present it in a a permanently findable format with nice large graphics, and maybe a short explanatory video.
More useful references:
http://oystein-debate.blogspot.com/2011/03/steven-jones-proves-primer-paint-not.html
So I'd like to do the same with one of the most popular claims - that thermite was found in the remains of the towers. Specifically with this 2009 paper:
http://www.911research.wtc7.net/mirrors/bentham_open/ActiveThermitic_Harrit_Bentham2009.pdf
I took some steps along this road with the post discussing the misidentification of "abundant manganese" via X-EDS, which taught me a little about the X-EDS process and problems. Jones, it seems, had difficulty correctly interpreting X-EDS back in 2006. Perhaps there were similar errors in the 2009 paper?
Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center Catastrophe
Abstract: We have discovered distinctive red/gray chips in all the samples we have studied of the dust produced by the destruction of the World Trade Center. Examination of four of these samples, collected from separate sites, is reported in this paper. These red/gray chips show marked similarities in all four samples. One sample was collected by a Manhattan resident about ten minutes after the collapse of the second WTC Tower, two the next day, and a fourth about a week later. The properties of these chips were analyzed using optical microscopy, scanning electron microscopy (SEM), X-ray energy dispersive spectroscopy (XEDS), and differential scanning calorimetry (DSC). The red material contains grains approximately 100 nm across which are largely iron oxide, while aluminum is contained in tiny plate-like structures. Separation of components using methyl ethyl ketone demonstrated that elemental aluminum is present. The iron oxide and aluminum are intimately mixed in the red material. When ignited in a DSC device the chips exhibit large but narrow exotherms occurring at approximately 430°C, far below the normal ignition temperature for conventional thermite. Numerous iron-rich spheres are clearly observed in the residue following the ignition of these peculiar red/gray chips. The red portion of these chips is found to be an unreacted thermitic material and highly energetic.
I recognize this is old ground, however my goal here is to produce a go-to debunk of the paper using past, synthesizing past debunks with any more recent information, and presenting it in an accessible manner.
So let's begin by gathering the past debunks, much of which was done deep within the JREF/ISF forum.
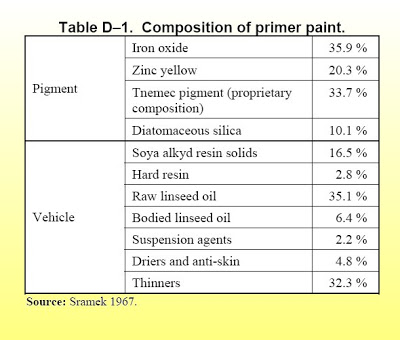
SE Jones, crawling under the name of Niels Harrit as lead author, published their study "Active thermitic material found..." of red-gray chips in 04/2009 in a pay-to-publish "paper" of Bentham publishers. Within days, debunkers had noticed that the hexagonal platelets in some of the chips is almost certainly kaolin clay, an aluminium silicate, which is essentially inert, of no use in any thermite preparation, and an ingredient of many paints. Also, it was clear almost immediately that the rhomic particles of hematite are simply red pigments - the very thing that makes steel primer paint red.
In 2010 or 11, Sunstealer at the JREF forum found an SE Jones presentation with XEDS analysis of actual paint from the external columns of the twin towers - it was a very good match with the red-gray chip from the Bentham paper that they soaked in MEK solvent to, purportedly, show that the aluminium is separate from the silicon - figur 14 in the Bentham paper. That paint ("Tnemec Red") has no aluminium silicate (such as kaolin) in its recipe - and that chip also was no match at all for the chips (figures 6 to 11) that contained the kaolin plates.
In summer 2011, the late Czech chemist Ivan Kminek discovered a second, different paint recipe used in the twins: The shop primer of LaClede steel manufacturer, who made the floor trusses.
This recipe does contain aluminium silicate, and simulated XEDS charts of that recipe are an excellent match for Harrit's and Jones's figures 6-11!
In early 2012, James Millette sent his analysis report of red-gray chips from another batch of WTC dust samples to Chris Mohr of the then JREF/today ISF forum. Clearly, the chips that best matched the Harrit/Jones main chips contain kaolin, hematite pigment, and epoxy binder - just like the LaClede primer.
That is the last achievement in this issue so far. Current status: Chips are primer paint - very little doubt.
There are small problems with Millette's report:
1. The chips I mentioned do not show a trace of strontium in Millette's analysis, as they ought to, if they are LaCLede primer - the recipe calls for a small amount of strontium chromate primer. However, we know from two of Jones' co-authors, Niels Harrit and Jeff Farrer, that THEY found strontium and chromium!
2. Millette didn't try to identify any of the other of various different "kinds" of chips, that quite certainly represent different paint recipes
Oy's summary likely refers to these thread on ISF (formally JREF)
- WTC Dust Study Feb 29, 2012 by Dr. James Millette
- Thread to Discuss The Excellent Analysis of Jones latest paper . [Sunstealer analysis]
- Chris Mohr's YouTube Part 23 Epilogue: WTC Dust Update; Saying Goodbye to 9/11 Truth
- The sad case of Niels Harrit
So what I'd like to do is create a simple yet deep summary of the evidence for paint, and present it in a a permanently findable format with nice large graphics, and maybe a short explanatory video.
More useful references:
http://oystein-debate.blogspot.com/2011/03/steven-jones-proves-primer-paint-not.html
Thursday, March 31, 2011
Steven Jones proves primer paint, not thermite
In their paper "Active Thermitic Material Discovered in Dust from the 9/11 World Trade Center Catastrophe"[1] of April 2009, the authors sought to identify the chemical nature of tiny "red-grey chips". In November 2009, one of the authors, Steven E. Jones, presented new data in Sydney, Australia[3]. This new data proves that the authors had looked at two different materials, and that one of them is primer paint from WTC steel. At the same time, it invalidates two of the main conclusions of the paper, namely Conclusion 3 ("Elemental aluminum became sufficiently concentrated to be clearly identified in the pre-ignition material") and Conclusion 6 ("From the presence of elemental aluminum and iron oxide in the red material, we conclude that it contains the ingredients of thermite."), as well as the main conclusion ("we conclude that the red layer of the red/gray chips we have discovered in the WTC dust is active, unreacted thermitic material...")
Attachments
Last edited: