Chemtrail Kook
New Member
Aluminum Barium Strontium -Mount Shasta snow pack readings - and soil samples from all over the world - all is well... don't mind the man behind the curtain...
[h=1]New tests find trace or no aluminum in area water[/h]Recent testing of the water in and around the city of Mount Shasta by a group of local citizens claims to have found levels of aluminum that are a danger to health.
Independent testing of several area water sources and the city’s water supply have found either no aluminum or trace amounts.
This is an interview I did with We Are Change Hawaii. Please watch, share and spread!!!!!
http://www.youtube.com/watch?feature=player_embedded&v=5ucUfw7u_kU
Someone created an account on ATS just to post this:
http://www.abovetopsecret.com/forum/thread703141/pg17#pid11344297
To which I replied:
Yet another thing that people can bring up is MJM ever gets around to answering his critics.
It's interesting that this was a new user. It makes me think that someone read the rather active thread there, and told the WITWATS crowd, and they send someone over there. I strongly suspect that they actually believed it was metallic aluminum. So their entire film is based on high-school-chemistry level mistake.
What is being found i snow on mountain tops around the world , and in the most documented case which is Mt Shasta california is FREE PARTICILOID METALIC ALLUMINIUM !!!
[...]
EPA testing and in particular 60108 is a test to measure harmfull levels of METALIC ALUMINIUM in test samples, not harmless aluminium oxides or silicates.
Yet another thing that people can bring up is MJM ever gets around to answering his critics.
Quoting that in full, because it it's WRONG. It's an understandable mistake, and quite possibly one that Michael J. Murphy made in his film What In The World Are They Spraying.
EPA 6010B is the test used in the samples that are discussed in the film, including the shasta test. It's "inductively coupled plasma-atomic emission spectrometry" (ICP-AES), which "determines
trace elements, including metals, in solution":
The relevant part of ICP-AES works like this:
A peristaltic pump delivers an aqueous or organic sample into a nebulizer where it is changed into mist and introduced directly inside the plasma flame. The sample immediately collides with the electrons and charged ions in the plasma and is itself broken down into charged ions. The various molecules break up into their respective atoms which then lose electrons and recombine repeatedly in the plasma, giving off radiation at the characteristic wavelengths of the elements involved.
So, yes, it determines the metals, but only after the sample has been broken down into a plasma of its atoms. No molecules exist. So the 7% of aluminum in dirt would show up as aluminum in this test.
If you are still unconvinced, then consider that it's the same test used for barium and strontium, and hopefully nobody is suggesting that there is metallic barium or strontium in the water samples, as that's impossible. Here's what happens when you add those to water:
It's all yet another example of how WITWATS is based on a misunderstanding of science. This is all basic high-school chemistry, yet they continually get it wrong. It's almost as if they are doing it deliberately.
Geological Society of America (1969) said:The notably high strontium content (1400 ppm) in the andesites from Mount Shasta also indicates a mantle origin at depths where plagioclase is absent as a stable phase in the residual material.
http://gsabulletin.gsapubs.org/content/81/1/311.abstract
Magna said:Abstract
We present lithium (Li) abundances and isotope compositions for a suite of anhydrous olivine tholeiites (HAOTs) and hydrous basalt-andesitic (BA) lavas from the Mt. Shasta and Medicine Lake regions, California. The values of δ7Li vary from +0.9‰ to +6.4‰ and correlate inversely with distance from the trench. These data are consistent with continuous isotope fractionation of Li during dehydration of the subducted oceanic lithosphere, an interpretation corroborated by uniformly high pre-eruptive H2O contents in basaltic andesites accompanied by high Li, Rb, Sr, Ba and Pb abundances. The subduction-derived component that was added to these hydrous magmas is shown to be very similar beneath both Mt. Shasta and Medicine Lake volcanoes despite characteristically distinct Li isotope compositions in the magmas themselves. More evolved andesites and dacites from Mt. Shasta have δ7Li from +2.8 to +6.9‰ which is identical with the range obtained for HAOTs and BA lavas from Mt. Shasta. Therefore, Li
isotopes do not provide evidence for any other crustal component admixed to Mt. Shasta andesites or dacites during magmatic differentiation and magma mixing in the crust."
http://web.mit.edu/people/tlgrove/p...d=501045&md5=5f6cb7f0833859c025710a3c2c04623b
INCREDIBLE Ph REPORTS this version 10/30/09
The soil scientists from the USDA Soil Conservation Department visited private property east of Shasta Lake, California, on October 27, 2009. Mr. Bailey, Komar, and Owens tested the pH with standard federal meters. All agreed the pH should be 5.5.
Under Douglas fir, the ph was 7.4, astoundingly basic for that habitat.
Under Ponderosa pine, at the precise soil-needle interface, I would expect a pH of 5. At that point, Bailey’s meter showed 6.5. This is high for a microhabitat that should be very acid. Old soil surveys indicate this soil should be very acid, around pH of 5.5.
I bought a house in Mt. Shasta old black oak/pine pasture in 2002, tested the pH at below 6, good for vegetable gardening. It was a major reason for purchase, and proceeded with highly acid composting of leaves and grass to drive the pH down or at least keep it low, as every master gardener knows. I added a touch of sulfur and avoided wood ash to insure acidity, and proceeded to teach organic gardening courses out of my yard though COS. The pH tests were an embarrassment because now my garden is pH 7, sometimes higher. This is the opposite of what should happen.
The pH meter of Jon McClellan proceeded to show pH in McCloud gardens also running close to 7 or 8, which is too high for heavy organic mulch with no ashes. General lawns were also running over pH 7 under oaks and pines and fir trees. This is contrary to everything I learned in college and the Soil Conservation Service for 35 years. The old data sheets say these soils should be running a pH of 5-6.
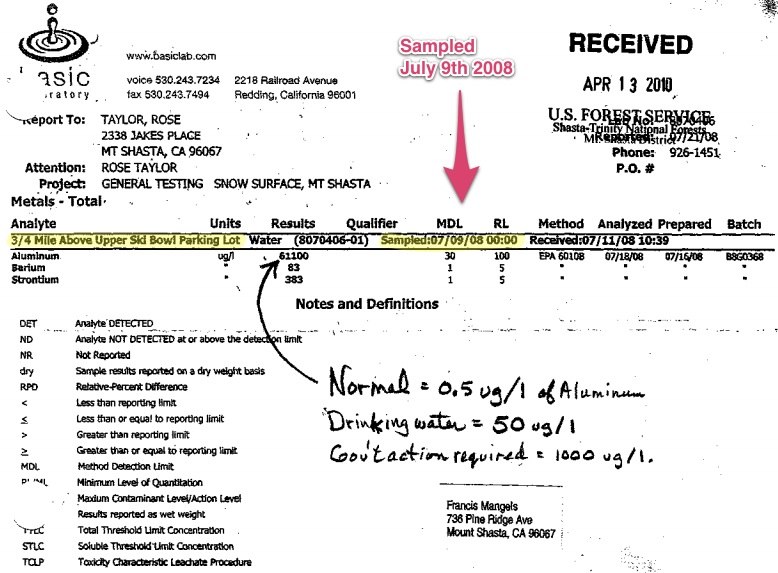
I tested my rainwater in a plastic NWS rain gauge set high on a pole, and got 1010 ug/l aluminum, with substantial amounts of barium and strontium included, where it should be non-detectable. Others from the West Coast have similar repeated results, from the Bay Area to Washington. The McCloud/Mt. Shasta area got snow at 61,000 ug/l, and a Shasta Lake pond got 375,000 ug/l of aluminum. EPA-approved lab tests are true.
The situation is widespread, so it is not a point source. At night you see a whitish gray sheen on a car window with a flashlight. Astronomy clubs get “night sparkles” in laser lights. It’s too thick to be Chinese. Something is coming down in a rain of dust, and it looks like aluminum so fine you can’t normally see it.
I am concerned about our lungs and asbestosis/asthma. Autism. Alzheimer’s. Drought enhancement. Our agricultural soil is degrading and trees are dying from bad fungi, bacteria, or lack of good ones. As a farmer told me, “The bugs are the wrong kind.”
Francis Mangels
ONGOING PH STUDIES OF SOILS AND WATERS IN NORTHERN CALIFORNIA FOLLOWING STRATOSPHERIC SPRAY OPERATIONS (GEOENGINEERING)
PH investigations are in full swing here.
I am using 5.5-8.0 pH hydrion lab papers to see what's going on around here.
Bottom lines:
I am using the papers correctly. Actually, they are practically foolproof.
Very little training needed. Used them all the time in college. Easily
available. Cheap. Fun, actually.
I have tested from Weed to Redding, in streams, muddy soils, gutter wash,
fresh rain, and even local tap water. I also cross-checked a few things
like orange juice, saliva, mucus for comparisons and got expected results to
prove the papers are working correctly.
Natural pH of rain, snow, forest floor, forest soils, under black oak or
mixed conifer, my garden, compost piles, city tree soils, should be about
5.5-5.6 or close to it.
The whole outside world is almost uniformly 6.5 to 6.8 pH. It is so damned
similar it is scary. Something is wrong with our ecology. My hypothesis is
that the outside world is so saturated with jet spray rain aluminum hydrox
and barium oxides that we are now fairly permeated to form an irreversible
higher pH. We have lost our natural acidity. I expect this to climb higher
into neutral 7.0 this summer, which is where the gardens were last summer.
I will still have a garden, but my acid loving crops have certainly declined
over the years, especially since 2007. No more potatoes, fewer tomatoes,
even with good compost.
Like the chief man in geoengineering at San Diego said, "I have absolutely
no idea what the long term effects will be on anything!" The human race is
now its own guinea pig.
I want an EIS done. For God's sake will somebody at least LOOK at the pH
side effects?
Francis Mangels
Water Technology Online said:Q:Our snow on Mount Shasta Mountain, Calif., has recently been tested by EPA as having 61 ppm aluminum, 0.08 ppm barium and 3.0 ppm strontium. These elements are apparently oxides on micron-sized flakes. Will this get into our groundwater supply? Will a porous sandy silt no-clay soil filter this stuff out?
Will this affect the bottled water plant here using Mt. Shasta city water? One of the others would not build here.
Will this have an effect on health if hikers melt and drink this water?
— Mt. Shasta, Calif.
A:Aluminum is listed in the EPA Secondary Drinking Water Standards, and as such, it is not considered a health hazard. The recommended level is 0.2 mg/L and concentrations above that are said to impart color to water, but are not a health concern.
Strontium, when expressed as ppm, is usually a metal that can be found naturally as a non-radioactive non-regulated element. Other naturally occurring strontium is found as four stable isotopes: Sr-84, -86, -87 and -88. Of greater concern is another isotope, strontium-90, the most prevalent radioactive isotope in the environment, although strontium-89 can be found around reactors, and strontium-85 is used in industry and medicine.
The U.S. Environmental Protection Agency (EPA) has set a MCL (Maximum Contaminant Level) for Sr-90 (and other radionuclides) that may be present in public drinking water. The MCL for beta emitters like these is 4 millirem per year or 8 picocuries per liter of water, and a water analysis would report the results in these units.
If the test for strontium is expressed as ppm or micrograms/liter, then the strontium is probably non-radioactive.
The EPA MCL for barium is 2 ppm. So, your snow melt contains less than all these MCLs that we’ve discussed but we don’t know what the city water contains.
It seems to me that you should be looking at your Mt. Shasta city water report. They are required to send this to the community once or twice per year. This is the water that a bottler should really be concerned about.
For drinkers of melted snow, the three contaminants we’ve just discussed don’t seem to be a problem.
David M. Bauman, CWS-VI, CI, CCO, is technical editor of Water Technology®and a water treatment consultant in Manitowoc, WI. He can be reached by e-mail at: dp-bauman@sbcglobal.net





2011 May 26 RealityZone
G Edward Griffin
It seems that the die-hard skeptics refuse to believe what they see with their own eyes. No matter how many laboratory tests we collect, they always seem to come up with a theory that, no matter how far fetched it is, would explain the high levels of aluminum, barium, and strontium as merely due to some climate condition or error in preparing the chemical sample or some unintended human interaction.
SKI SLOPE THEORY
When we released our documentary, What in the World Are They Spraying, we included snow samples taken from Mt. Shasta in Northern California, which contained toxic levels of these metals. Since snow in merely frozen rain water, it was clear that this came from the sky and not from the soil or water run-off from some toxic waste dump. Nevertheless, an Internet debunker challenged our conclusion by claiming that people ski on Mt. Shasta, and skis are made of aluminum. Therefore, the tested aluminum probably came from the skis! Nothing to worry about after all.
Of course, this was all made-up nonsense. People do ski on Mt. Shasta, but it is a big mountain, and there has never been any skiing in the area where the samples were taken. Even if there had been, that would not explain the high levels of barium and strontium. These metals are not used in the construction of skis. Our debunker never bothered to check on any of that. He was merely looking for some plausible explanation in order to plant doubts into the minds of casual readers. If people are confused by seemingly plausible explanations that even remotely could explain away the high levels of aluminum, barium, and strontium in snow and rain water, they will back away from coming to a conclusion and align themselves with the prevailing view.
DUST-BOWL THEORY
Another debunker contacted me a few days ago and claimed that a plausible explanation for the chemicals in snow on Mt. Shasta is that the samples were taken in a year with early snow melt which, according to him, means there was a lot of bare earth exposed at the time, and the wind must have blown dust from the earth onto the snow. Furthermore, he claims that the soil on Mt. Shasta contains the same metals as found in the samples; so, you see? Here is another perfectly plausible explanation. Once again, nothing to worry about.
We are planning to respond to this gentleman as soon as we can find the time to carefully examine his claims about the early snow melt, the amount of bare earth exposed, the composition of the surface soil, and especially the rainfall and moisture levels of the soil during this period. I expect to find that, even if there had been an early snow melt, the soil on Mt. Shasta would have been far too moist and covered with moss, ferns, or other ground cover to make the “dust-bowl” theory even remotely plausible. But that will take a little time to pull the facts together.
Meanwhile, we must not just play defensive and spend our lives answering the debunkers. We must take the initiative and obtain new data and information that will be impossible to dispute. The on-going collection of new snow and rain samples is part of that strategy. After we have literally hundreds of such chemical tests, I think our critics will run out of plausible-denial theories.

The site is owned by the owner of this quack medicine site: http://everythinghcg.com/
I spoke with Francis Mangels yesterday for about an hour. He has confirmed with a local geologist that I am 100% correct in my research on the soil around Mt. Shasta being naturally high in Aluminum, Barium, and Strontium.Statements by Francis Mangels:
During his interview with Food Integrity Now on March 7, 2011, Francis Mangels was asked if there was anywhere else aluminum in his samples could have come from, and he answered "no". The facts are, however, that Mt. Shasta and much of Siskiyou county is covered by remnants of the volcanic eruptions which formed the mountain 1/2 million years ago. Mangels knows this as he has mentioned in previous writings that the DEETZ soil series which covers much of the county.
Mangels "no" statement to Food Integrity Now:
http://foodintegritynow.org/2011/03/07/food-integrity-now-francis-mangels-chemtrails-e36/
Mangels opinion column in Mt. Shasta News quotes an unnamed source reiterating the same:
http://www.mtshastanews.com/opinion...ng-answers-to-aluminum-contamination-concerns
Mangels reference to Deetz series soils:
http://uncensored.co.nz/2011/03/07/chemtrail-lab-reports/
Location of the Deetz soil series(contains volcanic ash, most, base saturated, naturally contains aluminum oxide):
http://www.cei.psu.edu/soiltool/semtool.html?seriesname=DEETZ
The main types of ash from Mt. Shasta are andesite and dacite.
http://www.siskiyous.edu/shasta/geo/pro.htm
Mt Shasta Andesite is rich in aluminum, the mafic ash tends to be high in trace elements, including Ba.
http://petrology.oxfordjournals.org/content/51/7/1571.abstract
The andesite found at Mt. Shasta is known to be high in strontium.
http://geology.gsapubs.org/content/35/1/e149.full
"The notably high strontium content (1400 ppm) in the andesites from Mount Shasta also indicates a mantle origin at depths where plagioclase is absent as a stable phase in the residual material."
http://gsabulletin.gsapubs.org/content/81/1/311.abstract
"These data are consistent with continuous isotope fractionation of Li during dehydration of the subducted oceanic lithosphere, an interpretation corroborated by uniformly high pre-eruptive H2O contents in basaltic andesites accompanied by high Li, Rb, Sr, Ba and Pb abundances."
http://web.mit.edu/people/tlgrove/pubs/138-Magna.pdf
Conclusions:
Either Francis Mangels was unaware that Aluminum,Barium, and Strontium rich soils make up his county, he forgot about the facts he knew, or he intentionally did not mention these widely known facts. I note that Mangels is described as a USDA scientist with many years residence in Siskiyou county, and find it unlikely that such a person with that sort of experience and interest in the subject does not know the facts and did no research before drawing his conclusions. It appears that Michael J. Murphy has been relying on faulty information either way. He knows now, however, and I will await a reevaluation of his position.
=========================================
Statement by Michael J. Murphy:
During the Food Integrity Now interview of May 18, 2011, I mentioned that the Mt. Shasta News had reported two years ago that local tests found only trace or no aluminum in water samples of the area. This is important because these local tests had to have been known to Wigington, Mangels, and Rose Taylor prior to creation of Murphy's film, yet no mention of it had ever been made. Mangels and the others even asked for the local tests to be made! In his response, however, it was clear that indeed Murphy was aware of the local testing that contradicted the tests shown in the movie. Murphy claimed in the interview that the tests that contradicted his own were only from "well water." Murphy's claim is not true.
Wigington, Mangels, Taylor and Peterson ask local government to do testing confirming their own tests:
http://www.mtshastanews.com/opinion...ng-answers-to-aluminum-contamination-concerns
Two months later, this Mt. Shasta news article clearly shows a photo captioned "No aluminum was detected at the Mount Shasta city park headwaters, shown", and states, "• City Park headwaters - Not detected".
http://www.mtshastanews.com/news/x1176011800/New-tests-find-trace-or-no-aluminum-in-area-water
Conclusions:
Murphy is aware that the testing that Mangels, Wigington, and Rose Taylor requested had publicly contradicted those shown in his movie. Murphy nevertheless created the movie as if no such tests existed. Either Murphy never read the article, was misinformed about the article, or he intentionally did not mention the fact that further testing contradicted the movie and the so-called "evidence" he cites at every public appearance. He knows now, however, and I will await a reevaluation of his position.
• Ream Ave. and W.A. Barr Road pond - Not Detected;Mt. Shasta News said:"Water samples were collected April 20 and testing was done by Basic Labs in Redding using method EPA60108 with containers provided by the lab to ensure no contamination. The results are as follows:
• Ream Ave. and W.A. Barr Road pond - Not Detected;
• Shasta Ranch Road pond - 0.097 milligrams per liter;
• Sisson Meadows pond - 0.085 milligrams per liter;
• City Park headwaters - Not detected;"
Did Mangels say anything about the sample being taken in July?
I think Griffin would probably be interested in this, particularly based on his statement above:
We are planning to respond to this gentleman as soon as we can find the time to carefully examine his claims about the early snow melt, the amount of bare earth exposed, the composition of the surface soil, and especially the rainfall and moisture levels of the soil during this period. I expect to find that, even if there had been an early snow melt, the soil on Mt. Shasta would have been far too moist and covered with moss, ferns, or other ground cover to make the “dust-bowl” theory even remotely plausible. But that will take a little time to pull the facts together.
