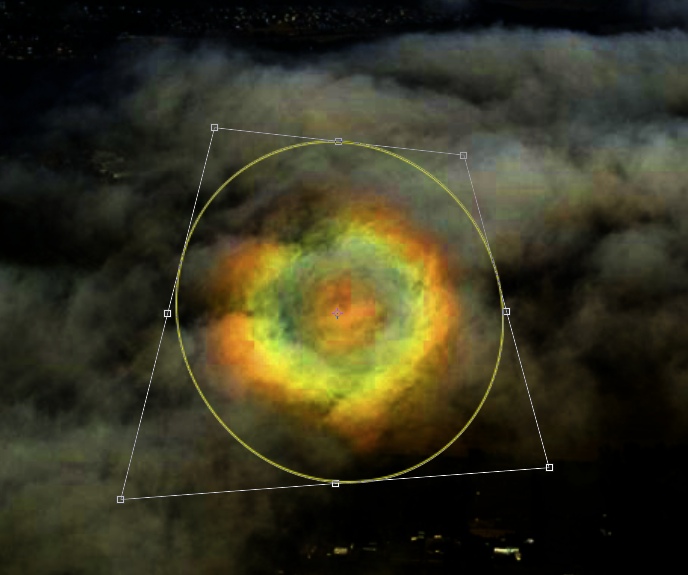
A recent post on The Ann Arbor News Facebook Page displayed a photo of a solar halo with some other clouds and some aircraft contrails

They included a short explanation of what it was:
1) Atmospheric Optical Phenomena such as Halos, Sun-Dogs, "Fire Rainbows", and iridescent clouds" have been seen for thousands of years, and recorded and understood for over a hundred years
2) They can only be made by clouds of ice water.
The Science and History of Halos, etc.
Haloes have been observed since ancient times. Here's a 17th Century painting of some haloes.

And here's a 1957 book showing a halo with some contrails contributing to it:

https://www.flickr.com/photos/metabunk/sets/72157642384424663/
Halos are formed by the refraction of sunlight through billions of tiny ice crystals in the air.
The colors in the halo occur because of refraction. This the bending of light as it goes through a prism. Different wavelengths (colors) of light are bent
different amounts, and this produces the spectrum of colors. We've all see this with the classic triangular prism splitting light.
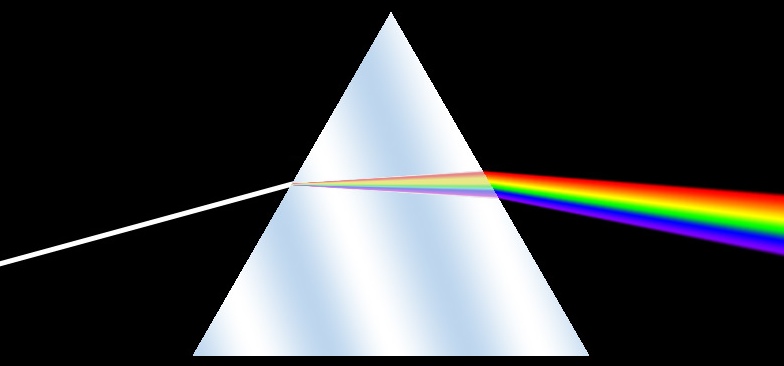
A hexagonal ice crystal is doing basically the same thing, as it's like a triangular prism with the corners cut off.
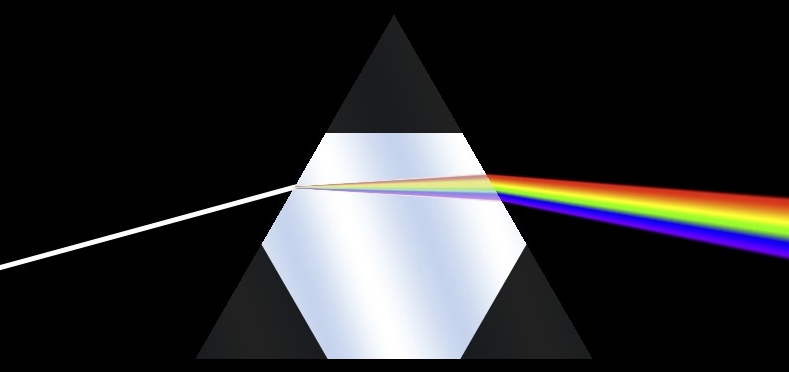
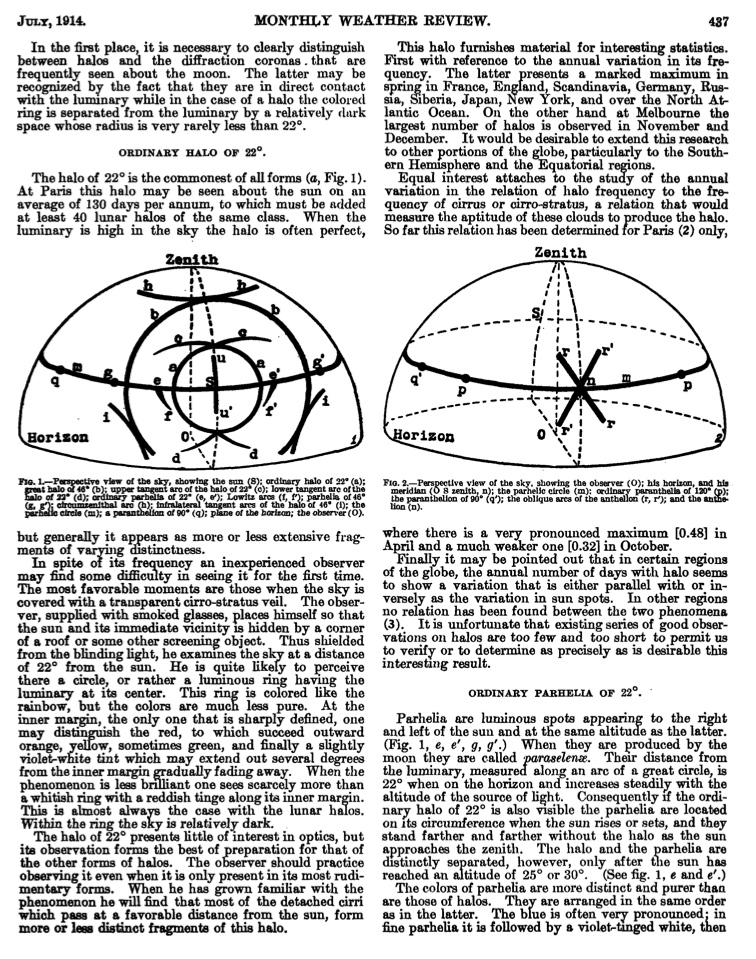
The most common form of Halo is the 22° halo, which is the one in the top photo. It's called a 22° halo because of the angle between the sun and the inner edge of the halo. This angle comes about in part because of the angle of the faces in the ice crystals.
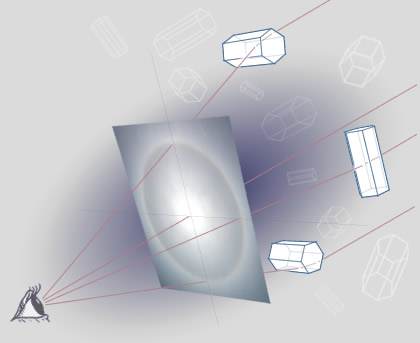
http://www.atoptics.co.uk/halo/circ2.htm
Why it can only be water
There are several primary reasons why only water can make these halos.
1) Hexagonal transparent crystals
2) Refractive Index
3) Quantity Required
4) Temporary nature
1) Hexagonal transparent crystals
The 22° Halo requires transparent faces which are at 60° to each other. You can only get this with a triangular or hexagonal crystal structure. Water forms hexagonal shapes naturally. No chemicals form triangular crystals. So we can eliminate any crystal substance that does not have a natural sixfold symmetry, such as common table salt crystals:

We can also eliminate any finely milled substance, which would produce irregular shapes with rough surfaces incapable of refracting light. Here, for example, is a fine dust (nano scale on the right) of Aluminum Oxide:
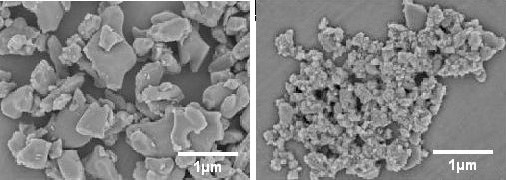
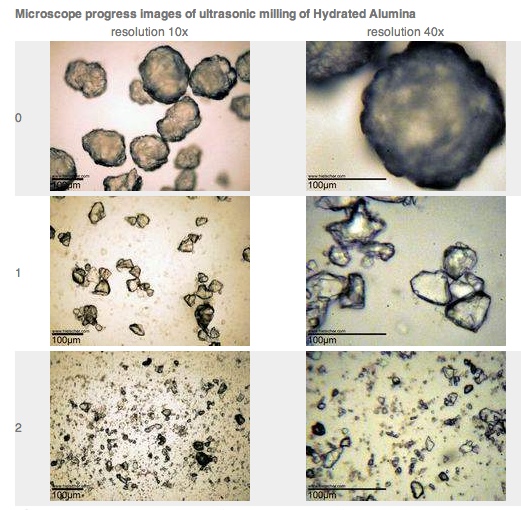
And here's a more typical milled alumina dust:
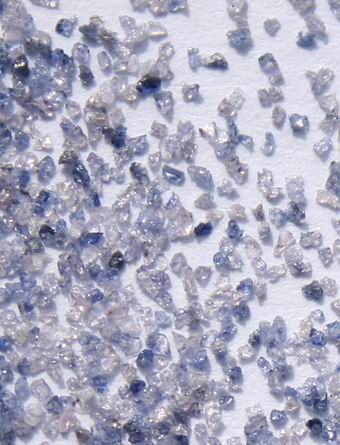
We can also eliminate commercially manufactured Alumina power, the purest form of which is known as mono-crystalline aluminum oxide, and looks like:
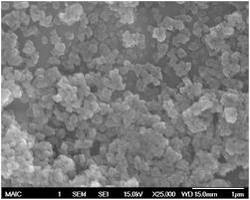
Or nano sized:
Irregular colored crystal will not make a halo.
We can also eliminate most naturally occurring minerals, as they generally contain impurities that make the crystal either opaque, or they will color or scatter the light, or both.
The crystals would have to be grown on site from a near 100% pure vapor - which of course is trivial to do with water - as it's the natural process of ice cloud (or ice fog) formation. But a lot harder to do with an artificial substance - especially hard to do with aluminum oxide, with a melting point of 3,762°F (2,072°C). And even then, while aluminum oxide does have some hexagonal geometry from some angles, it does not tend to grow into hexagonal shapes.
2) Refractive Index
Ice has a refractive index of of 1.31. Pure Aluminum Oxide crystals (Al2O3, Corundum) has a refractive index of 1.76. The refractive index governs the angle, and so Aluminum Oxide could not make a 22° Halo, because it would refract light at the wrong angle.
http://www.micro.magnet.fsu.edu/primer/java/prismsandbeamsplitters/equilateralprism/
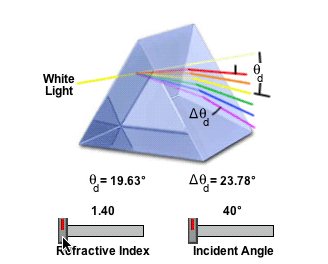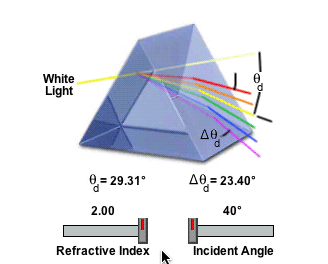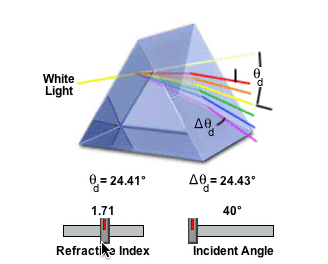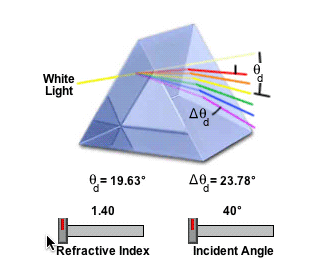
So if the halo is standard sized, then it's either water, or something with the same refractive index as (frozen) water.
3) Quantity Required
Clouds weigh quite a lot, even though they are not very dense, they are very big. Halos essentially form in a fog of cirrus. Cirrus clouds have about 0.03 g/m3 (grams per cubic meter) of water in them. Let's call that 0.01 for a fine ice haze. Now a 22 degree halo extends out a bit from the 22 degree, say 30 degrees. It' generally some around 12 km up, and the sun around 45 degree. This gives an actual circle of ice fog (12*tan(30)) = 7 km radius (being very rough with the geometry here). A thin layer of cloud would be 100m. So that 3.14*7000*7000*100 = 15386000000 cubic meters of ice fog. Multiply that by 0.03 gives 461580000g, or 461 metric tons.
And that's just if we've somehow just managed to weave the crystals into a small thin patch of sky directly in front of the sun. In the more normal case, the sky is covered with cirrus haze (say 50 km in each direction, which has a thickness of 1km or more. The amount of crystal needed to create such an cirrus overcast is over 200,000 metric tons. This is simply not possible to do with something like aluminum oxide.
So how is it possible with contrails? A typical plane creates 100-300 metric tons of water from the fuel. Yet an full sky overcast can occur with just a handful of planes. Where is the extra water coming from? The answer is it comes from the atmosphere. The air is at 70% relative humidity, meaning it's already holding millions of tons of water. The addition of the small amount of water from the engine pushes the relative humidity briefly over 100%, allowing the water to condense out in tiny drops, which then freeze. The ice crystals continue to grow from the atmospheric water, adding up to 1,000x their original weight.
That's simply impossible to duplicate by spraying a crystal powder. all you have is the original crystal, and that's nowhere near enough to cover the sky. A handful of planes would end up 1/1000th the optical density of the equivalent contrail-induced cirrus.
4) Temporary nature
Let's say you did somehow manage to loft up 200,000 metric tons of nano-manufactured perfect aluminum-oxide crystals, and ignoring the problems with geometry, and refractive index. Then the next problem is the temporary nature of solar halos. Sometimes they just last for a few minutes or hours. They are there one day, and almost always gone the next, and usually gone much sooner. Where does the overcast go?
The obvious answer with a cirrus haze is that it evaporates (technically it "sublimes", turning from solid back to water vapour). It does this quite naturally as the air heats up, or as the ice crystals grow large enough to slowly sink into slightly warmer air. It basically just fades away.
But if some other substance were being sprayed, then where does it go? How can it vanish in a few hours? Of course it can't. Water can vanish because it's basically being re-absorbed back into the atmosphere as water vapor. But a powder of aluminum oxide isn't going to vanish. It's just going to stay there, for many days, until it slowly sinks to the earth. But that's not what we see. So it must be water.

They included a short explanation of what it was:
Unfortunately the comments below the photo were quickly overrun by believers in the "chemtrail" theory (and then by other people trying to explain things to them). A few samples:Daniel Frei's Photography shared this photo with us of a 'sun dog' that was visible Saturday afternoon. Here's a little explanation from him: The ring is caused by sunlight passing through ice crystals in cirrus clouds within the Earth's atmosphere. Although more common in the spring and fall, this can happen anytime when the northern jet stream descends southward, drawing down Arctic air masses.
There's two equally important things you need to know about this story:
- The media is by far the most perverted evil for its continued deseption, These are Chemical trails!, Beware the pitch forks are coming,, and God is watching!
- Family worked on these military weather mod/manipulation programs for years. That entire picture contains end results of those type programs.
- This is caused by chemtrails/geoengineering this is not natural.
- Barium salts could easily mimic ice crystals,what do we find in our rainwater.... Aluminium, Barium, Strontium, arsenic & many other metals, just a coincidence???
1) Atmospheric Optical Phenomena such as Halos, Sun-Dogs, "Fire Rainbows", and iridescent clouds" have been seen for thousands of years, and recorded and understood for over a hundred years
2) They can only be made by clouds of ice water.
The Science and History of Halos, etc.
Haloes have been observed since ancient times. Here's a 17th Century painting of some haloes.

And here's a 1957 book showing a halo with some contrails contributing to it:

https://www.flickr.com/photos/metabunk/sets/72157642384424663/
Halos are formed by the refraction of sunlight through billions of tiny ice crystals in the air.
The colors in the halo occur because of refraction. This the bending of light as it goes through a prism. Different wavelengths (colors) of light are bent
different amounts, and this produces the spectrum of colors. We've all see this with the classic triangular prism splitting light.

A hexagonal ice crystal is doing basically the same thing, as it's like a triangular prism with the corners cut off.

The most common form of Halo is the 22° halo, which is the one in the top photo. It's called a 22° halo because of the angle between the sun and the inner edge of the halo. This angle comes about in part because of the angle of the faces in the ice crystals.

http://www.atoptics.co.uk/halo/circ2.htm
Why it can only be water
There are several primary reasons why only water can make these halos.
1) Hexagonal transparent crystals
2) Refractive Index
3) Quantity Required
4) Temporary nature
1) Hexagonal transparent crystals
The 22° Halo requires transparent faces which are at 60° to each other. You can only get this with a triangular or hexagonal crystal structure. Water forms hexagonal shapes naturally. No chemicals form triangular crystals. So we can eliminate any crystal substance that does not have a natural sixfold symmetry, such as common table salt crystals:

We can also eliminate any finely milled substance, which would produce irregular shapes with rough surfaces incapable of refracting light. Here, for example, is a fine dust (nano scale on the right) of Aluminum Oxide:

And here's a more typical milled alumina dust:

We can also eliminate commercially manufactured Alumina power, the purest form of which is known as mono-crystalline aluminum oxide, and looks like:

Or nano sized:

Irregular colored crystal will not make a halo.
We can also eliminate most naturally occurring minerals, as they generally contain impurities that make the crystal either opaque, or they will color or scatter the light, or both.
The crystals would have to be grown on site from a near 100% pure vapor - which of course is trivial to do with water - as it's the natural process of ice cloud (or ice fog) formation. But a lot harder to do with an artificial substance - especially hard to do with aluminum oxide, with a melting point of 3,762°F (2,072°C). And even then, while aluminum oxide does have some hexagonal geometry from some angles, it does not tend to grow into hexagonal shapes.
2) Refractive Index
Ice has a refractive index of of 1.31. Pure Aluminum Oxide crystals (Al2O3, Corundum) has a refractive index of 1.76. The refractive index governs the angle, and so Aluminum Oxide could not make a 22° Halo, because it would refract light at the wrong angle.
http://www.micro.magnet.fsu.edu/primer/java/prismsandbeamsplitters/equilateralprism/
The amount of light deviation by a prism is a function of the incident angle, the prism apex (top) angle, and the refractive index of the material from which the prism is constructed. As prism refractive index values are increased, so is the deviation angle of light passing through the prism.

So if the halo is standard sized, then it's either water, or something with the same refractive index as (frozen) water.
3) Quantity Required
Clouds weigh quite a lot, even though they are not very dense, they are very big. Halos essentially form in a fog of cirrus. Cirrus clouds have about 0.03 g/m3 (grams per cubic meter) of water in them. Let's call that 0.01 for a fine ice haze. Now a 22 degree halo extends out a bit from the 22 degree, say 30 degrees. It' generally some around 12 km up, and the sun around 45 degree. This gives an actual circle of ice fog (12*tan(30)) = 7 km radius (being very rough with the geometry here). A thin layer of cloud would be 100m. So that 3.14*7000*7000*100 = 15386000000 cubic meters of ice fog. Multiply that by 0.03 gives 461580000g, or 461 metric tons.
And that's just if we've somehow just managed to weave the crystals into a small thin patch of sky directly in front of the sun. In the more normal case, the sky is covered with cirrus haze (say 50 km in each direction, which has a thickness of 1km or more. The amount of crystal needed to create such an cirrus overcast is over 200,000 metric tons. This is simply not possible to do with something like aluminum oxide.
So how is it possible with contrails? A typical plane creates 100-300 metric tons of water from the fuel. Yet an full sky overcast can occur with just a handful of planes. Where is the extra water coming from? The answer is it comes from the atmosphere. The air is at 70% relative humidity, meaning it's already holding millions of tons of water. The addition of the small amount of water from the engine pushes the relative humidity briefly over 100%, allowing the water to condense out in tiny drops, which then freeze. The ice crystals continue to grow from the atmospheric water, adding up to 1,000x their original weight.
That's simply impossible to duplicate by spraying a crystal powder. all you have is the original crystal, and that's nowhere near enough to cover the sky. A handful of planes would end up 1/1000th the optical density of the equivalent contrail-induced cirrus.
4) Temporary nature
Let's say you did somehow manage to loft up 200,000 metric tons of nano-manufactured perfect aluminum-oxide crystals, and ignoring the problems with geometry, and refractive index. Then the next problem is the temporary nature of solar halos. Sometimes they just last for a few minutes or hours. They are there one day, and almost always gone the next, and usually gone much sooner. Where does the overcast go?
The obvious answer with a cirrus haze is that it evaporates (technically it "sublimes", turning from solid back to water vapour). It does this quite naturally as the air heats up, or as the ice crystals grow large enough to slowly sink into slightly warmer air. It basically just fades away.
But if some other substance were being sprayed, then where does it go? How can it vanish in a few hours? Of course it can't. Water can vanish because it's basically being re-absorbed back into the atmosphere as water vapor. But a powder of aluminum oxide isn't going to vanish. It's just going to stay there, for many days, until it slowly sinks to the earth. But that's not what we see. So it must be water.
Last edited: