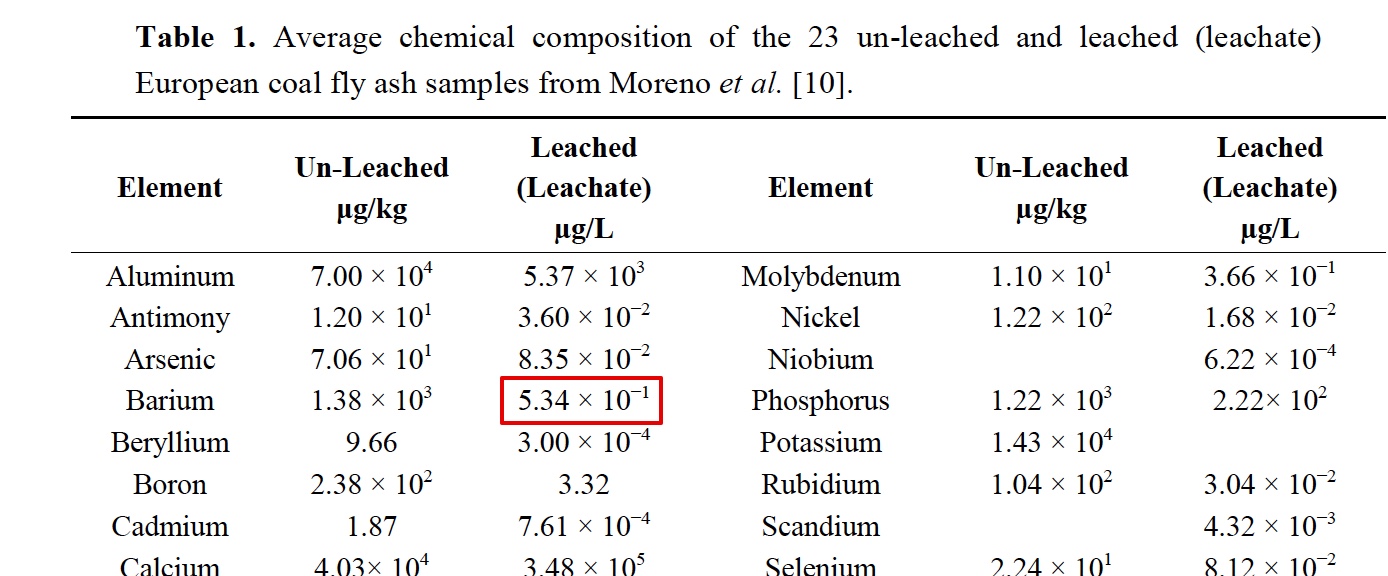
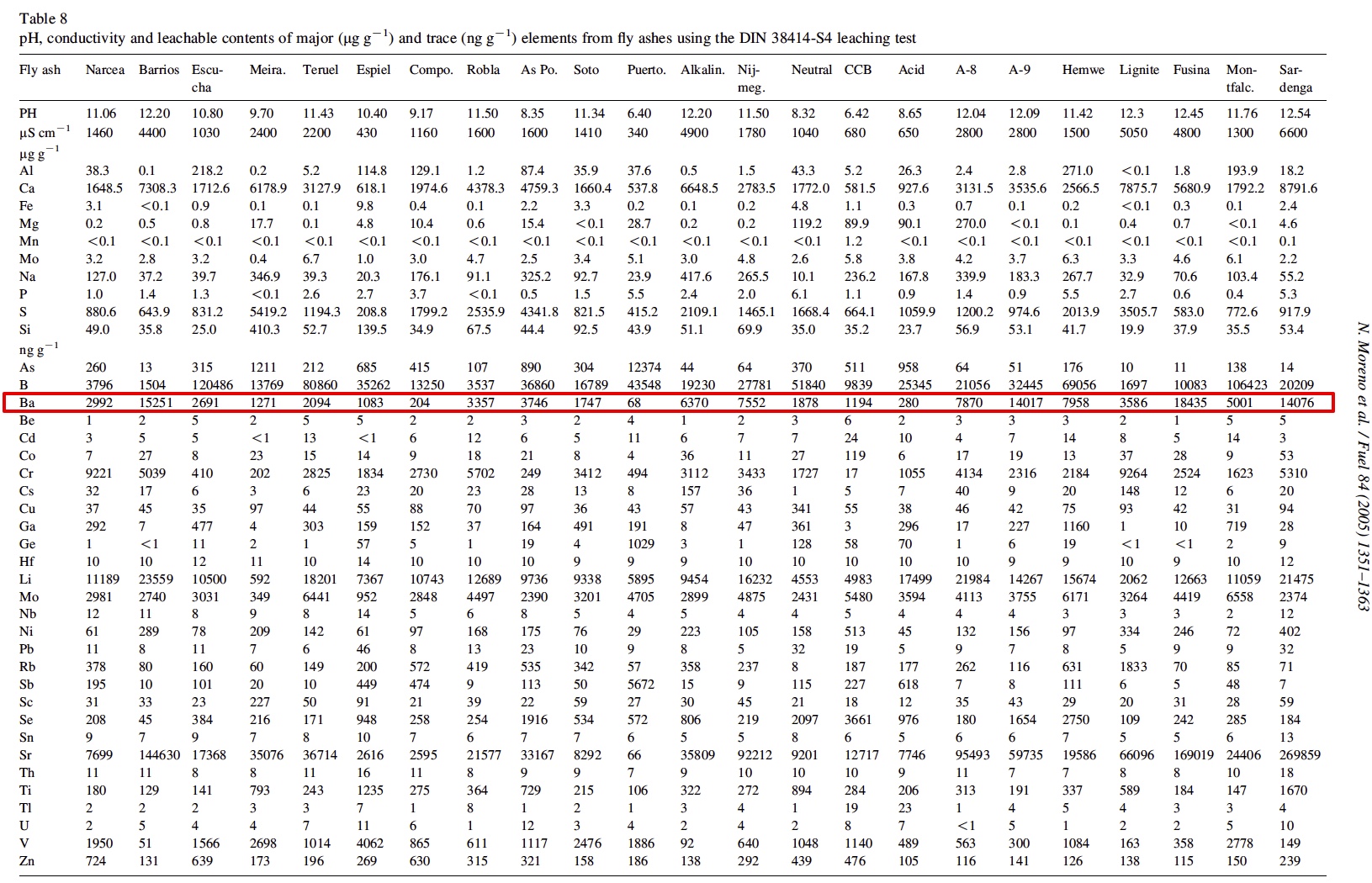
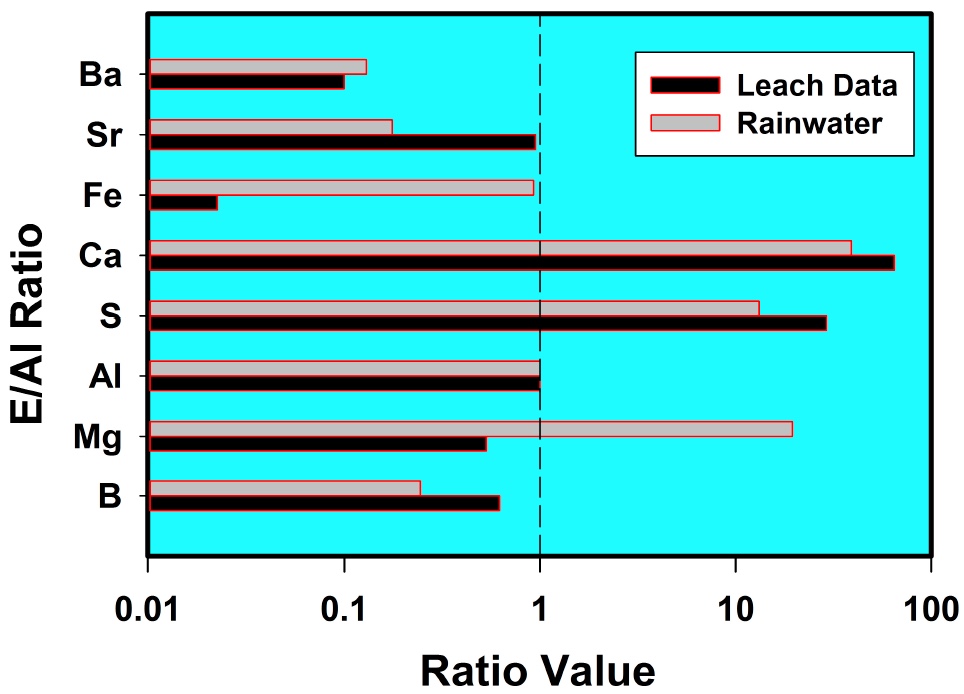
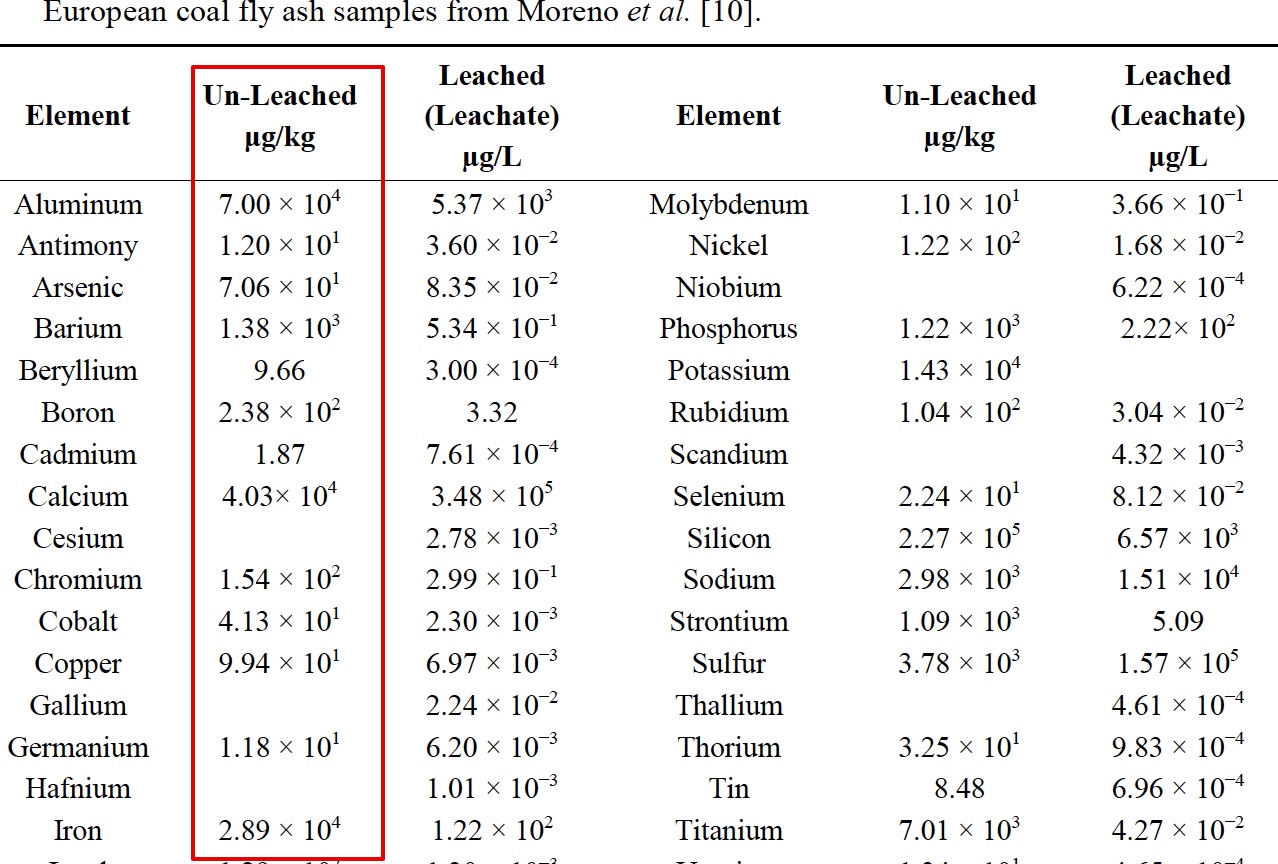
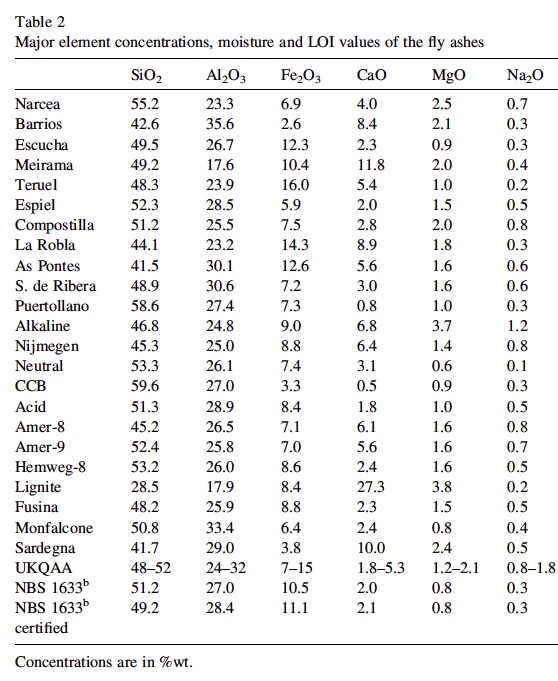
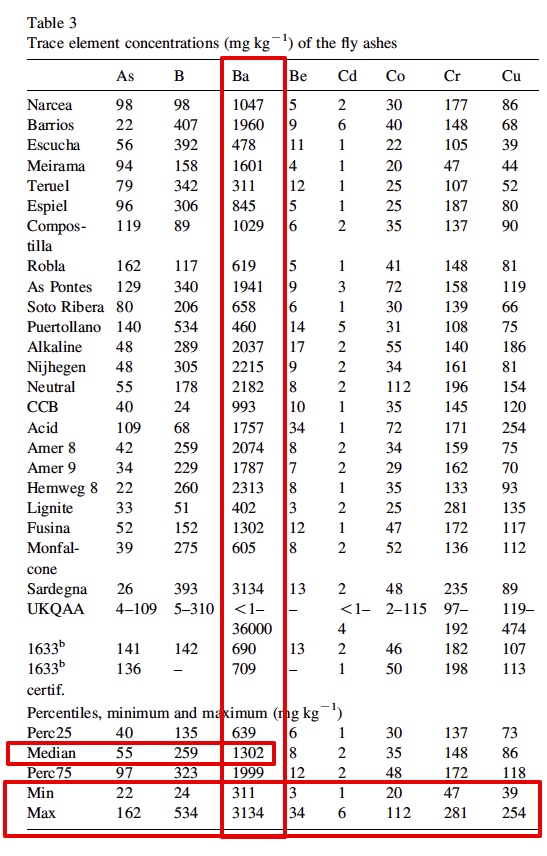
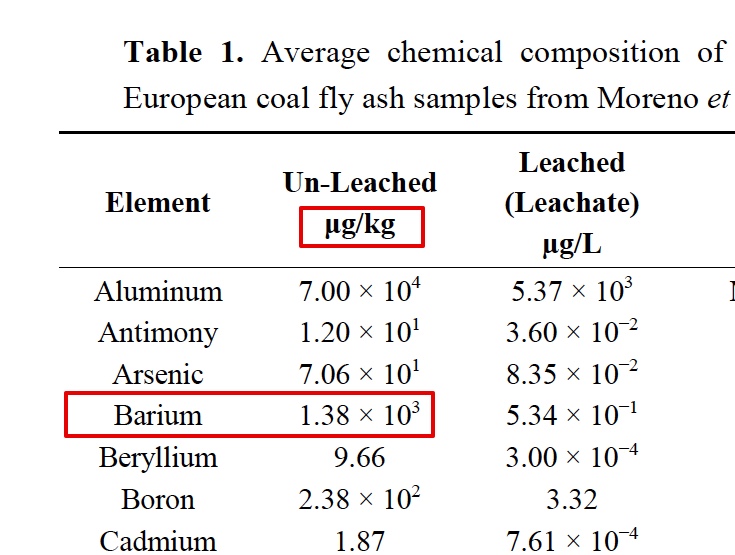
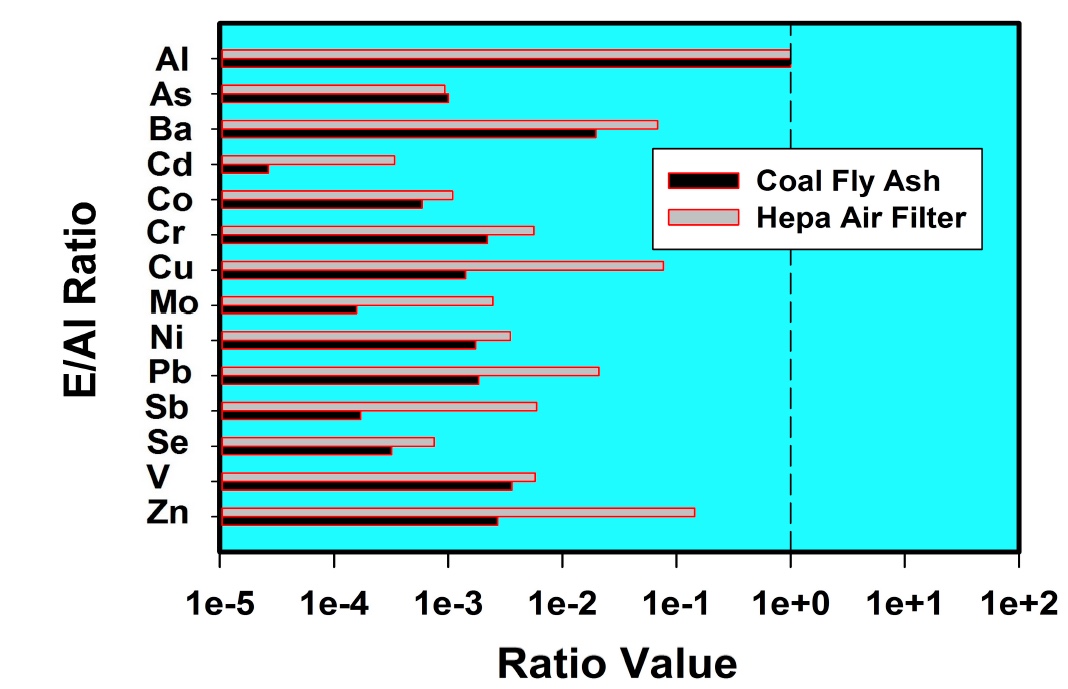
It is interesting that he only "began to notice" traile in spring of 2014. Supposedly the "program" has been going on for how long? Some people say the 50s, some the 90s. He says these are tanker jets but never says how he identifies them as tanker jets. That's enough for the chemtrail crowd though. They've posted the paper everywhere.
It seems the professor is not terribly observant. I just clicked a random point on a freeway in San Diego on Google Maps and looked back through the historical Street View imagery (of which there is a lot to choose from in this area).
This is from an image captured in October 2011:
The same spot in summer 2014, after the start of the alleged spraying campaign.