But when you put liquid water in space — where it can no longer remain as a liquid — which one of these two things happens? Does it freeze or boil?
The surprising answer is it does both:
first it boils and
then it freezes! We know this because this is what used to happen when astronauts felt the call of nature while in space. According
to the astronauts who’ve seen it for themselves:
When the astronauts take a leak while on a mission and expel the result into space, it boils violently. The vapor then passes immediately into the solid state (a process known as desublimation), and you end up with a cloud of very fine crystals of frozen urine.
There’s a compelling physical reason for this: the high specific heat of water.
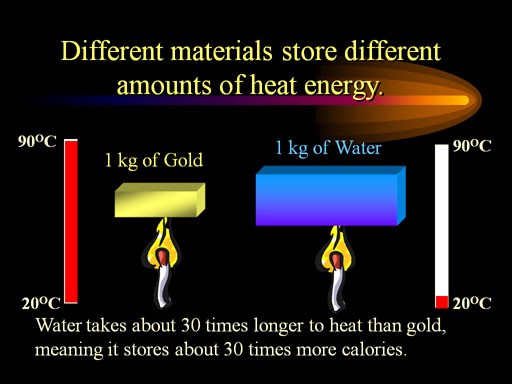
Image credit: ChemistryLand, via
http://www.chemistryland.com/CHM151S/06-Thermochemistry/Energy/EnergyUnitSpecificHeat.html.
It’s incredibly difficult to change the temperature of water
rapidly, because even though the temperature gradient is huge between the water and interstellar space, water holds heat incredibly well. Furthermore, because of surface tension, water tends to remain in spherical shapes in space (as you saw above), which actually minimize the amount of surface area it has to exchange heat with its subzero environment. So the freezing process would be incredibly slow, unless there were some way to expose every water molecule
individually to the vacuum of space itself.
But there’s no such constraint on the pressure; it’s effectively
zero outside of the water, and so the boiling can take place immediately, plunging the water into its gaseous (water vapor) phase!