I'll try, but I need more words:
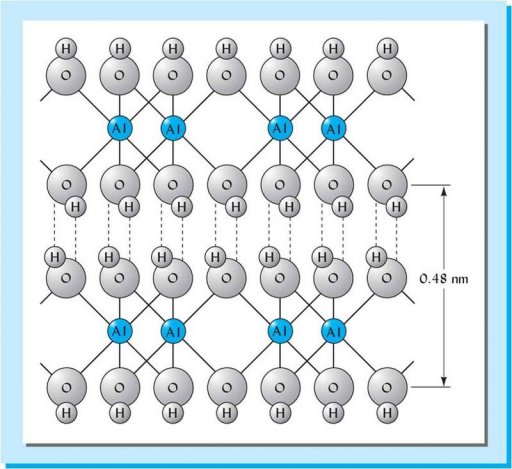
The atoms that are so nicely connected and ordered in the image of the clay structure will be cracked by immense heat for the analysis - all the connections are broken, the sample is literally
vaporized. No more clay - what's left are the loose atoms that once made it up.
Then there comes the trick: the vapor (that was clay once) is heated so much that it glows - it sends out light. If this light is split up into a spectrum (by sending it through a prism), the resulting "rainbow" has distinctive thin lines, like fingerprints, for each atomic element that was present in the vapor.
Wikipedia has a
nice example for helium:
View attachment 10256
In the case of the clay sample, there will be the lines of hydrogen, oxygen and aluminum.
BTW, the spectrum part of the process is
not really rocket science, but the heating and containment of tiny samples is a bit more intricate, as is the quantification of the elements detected.