Jazzy
Closed Account
Yes, a simple sword through the heart normally works.I'm all for KISS.
Yes, a simple sword through the heart normally works.I'm all for KISS.
"Lead in jet fuel" why when where? Citation?You keep saying contrails are caused by freezing water in airplane exhaust...
Since when did airplanes start using water for fuel? So even without additional geoengineering salts being sprayed behind a plane, what comes out the exhaust is definitely full of chemicals, not water.
Not too long ago they even put lead in jet fuel to make it burn at a higher temperature. So how do you really believe it is just harmless water, and not a chemical cocktail coating our sky? I would love to get my hands on a water powered car or airplane if you know how to make that happen!
"Lead in jet fuel" why when where? Citation?
Summary
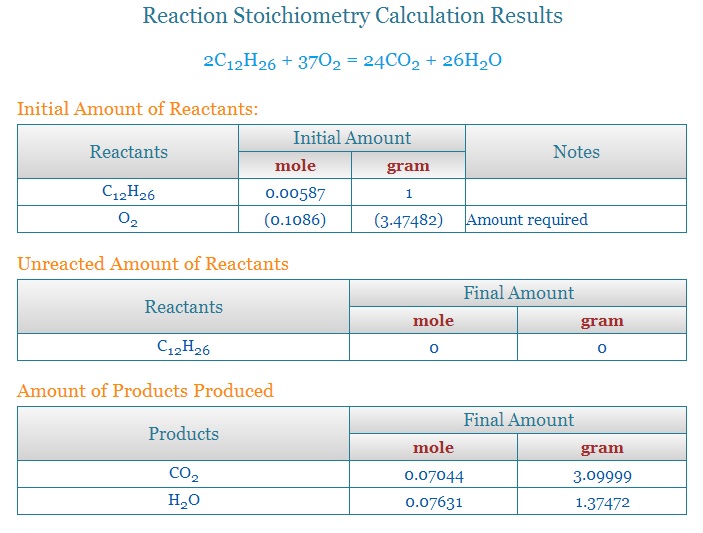
Kerosene is a fuel composed of a mixture of hydrocarbons with varying ratios of hydrogen and carbon between
C10H22(decane) and C16H34(cetane/diesel). The average kerosene would be C12H26. The ratio of fuel burned to water produced by these ranges are as follows:
C10H22 produces water at the ratio of 1.3931 units of water for every unit of fuel burned.
C12H26 produces water at the ratio of 1.37472 units of water for every unit of fuel burned.
C16H34 produces water at the ratio of 1.35365 units of water for every unit of fuel burned.
In a mile a jetliner uses at most 50 of fuel, most quite a bit less, per mile. That would make around 8 gallons of water. Will someone please show me, using the equations that describe the actions of water vapor in the atmosphere how that little amount of water can spread out to be 5 degrees wide at 36k feet(that would be an area .6 miles by one mile) and persist for 30 minutes in a clear sky? It must solved in the dynamic atmosphere.
You say that it is just water vapor, there are equations that describe the actions of water vapor in the atmosphere. Use science to show the amount of water vapor put out by a jetliner can act in the manner observed.
If you think my numbers are too large an area or long of a time, show how much area and how long a cloud could persist.
...there is no amount of a substance could be carried in payload which could account for the optical density of a persistent contrail. The only possible source for such a mass is accretion of water vapor from the air onto the contrail particles formed by the exhaust.
Here is an early reference predating 'chemtrails' from the 1970's which found that the ice budget shows an aged persistent contrail contained four orders of magnitude (104 or 10,000 times more) mass than the original ice mass generated by combustion: http://journals.ametsoc.org/doi/pdf/10.1175/1520-0469(1972)029<1367:MOTGOT>2.0.CO;2
No such supply of supposed chemtrail chemicals exist in that same atmosphere, so how do chemtrails expand and thicken?
or some very reaching references to the idea of moisture-absorbing substances (I forget what that is called, technically).
How did you reach this conclusion?Even with short contrails the majority of the water in the trail you see comes from the atmosphere. .....
How did you reach this conclusion?
Via lack of coffee. I was thinking of the case when the humidity is JUST too low for contrails to persist, but even then the atmospheric water will only be the majority at certain temperatures.
So in a non-persistent contrail, the majority of the water still might be atmospheric, but probably not.
I think it is somewhat moot if a particular water molecule in an ice contrail came from the engine or the surrounding air. There is no net mass increase in the mass of a short contrail (RHi <100%) due to water from the surrounding air, above that from the engine exhaust alone. .IMO
Just for the layman's sake (like me).. how can you have more than 100% humidity? Is that referring to greater than 100% in that specific localized streak where the aircraft is flying the RHI is temporarily raised over 100% behind the engine; or is that more in reference to overall humidity at altitude?
*Edit - I apologize for that nasty run-on.. just couldnt think of a better way to be specific enough for definition.
Is that referring to greater than 100% in that specific localized streak where the aircraft is flying the RHI is temporarily raised over 100% behind the engine; or is that more in reference to overall humidity at altitude?
And in my post above, normally I'm referring to RHI>100% in the ambient air - so the air is persistent contrail friendly. But here I'm talking about short contrails, and the brief time while the air and exhaust are still mixing. The ambient air is <100% RHi, but the mixture is >100% RHi for a few seconds, allowing the ice crystals to increase in size.
And in my post above, normally I'm referring to RHI>100% in the ambient air - so the air is persistent contrail friendly. But here I'm talking about short contrails, and the brief time while the air and exhaust are still mixing. The ambient air is <100% RHi, but the mixture is >100% RHi for a few seconds, allowing the ice crystals to increase in size.
So if a cubic meter of air was capable of holding 100 grams of water
As this graph shows, at -40C the air can hold only a tiny amount of water
" you remove all the air from the given volume, nothing changes" Not quite "nothing", the relative humidity changes, as does the mass mixing ratio.Note that the air itself has nothing to do with it. It's not the air that somehow "holds" water. It's the volume. If you remove all the air from the given volume, nothing changes. So phrases like "x amount of air can hold y amount of water vapor" are inaccurate.
No, the relative humidity would not change." you remove all the air from the given volume, nothing changes" Not quite "nothing", the relative humidity changes, as does the mass mixing ratio.
You are correct: relative humidity is defined as the ratio of the partial pressure of water vapour to the equilibrium vapour pressure over water (or ice, as appropriate), and by the ideal gas law, partial pressures are independent of the presence of any other gases (eg the air). So the air doesn't play any part in "holding the water", but I'm not sure how useful it is to make that distinction, considering that in the real world the air is there.Note that the air itself has nothing to do with it. It's not the air that somehow "holds" water. It's the volume. If you remove all the air from the given volume, nothing changes. So phrases like "x amount of air can hold y amount of water vapor" are inaccurate.
That is correct. If you removed just the air from the atmosphere, you wouldn't be left with a vacuum, and the oceans wouldn't boil away. You'd have a water vapour atmosphere containing exactly the same amount of water vapour there is currently in the atmosphere, and the equilibrium wouldn't change.No, the relative humidity would not change.
Skitt’s Law . :-(No, the relative humidity would not change.
Dear Mick, is there any reference for these figures? I saw them before, but couldn't find the source.
The first is from the IPCCDear Mick, is there any reference for these figures? I saw them before, but couldn't find the source.
My sincere gratitude for your help! Thank you very much!The first is from the IPCC
http://www.ipcc.ch/ipccreports/sres/aviation/index.php?idp=99
The second shows up in a variety of places. I think the original might be from dlr.de, or some German paper.
The basic numbers for water and CO2 come from the combustion equations.
Hi Mick, for comparison, is there a graphic in this series showing the 'no contrail' version? I can imagine how it looks and the dynamics, still I would like to see it and share all four. And I am also curious about #1-3 also, what do these show?Good point. The water in a jet exhaust is in the form of a superheated gas when it comes out. If you could just capture that gas, and keep it in one place, and let it cool down, then you would indeed always get a cloud.
However, the exhaust is mixing very rapidly with the external air. This mixing is what makes it cool down so quickly, but it dilutes the density of water vapor in the air.
At some point it's cold enough to condense out. But it's only going to condense out if the relatively humidity of the mixing air + exhaust goes above 100% at some point. So this is greatly affected by the existing water in the air.
This is why you keep seeing these graph. It shows the exhaust going from B to A as it mixes with the ambient air. B is the exhaust, always the same temp and water content. A (1, 2, and 3) is the ambient temp and humidity. See how a different value of A affects if a contrail forms or persists. (not shown is the case where no contrail forms at all, but you can work that out.

That would just be any line that does not go through the "Cloud" region.Hi Mick, for comparison, is there a graphic in this series showing the 'no contrail' version?
These? They are just explaining the chart.And I am also curious about #1-3 also, what do these show?
