Jazzy
Closed Account
The molten iron would have been blue/white had you been able to see it.thats clearly not the case that it would be blue/white
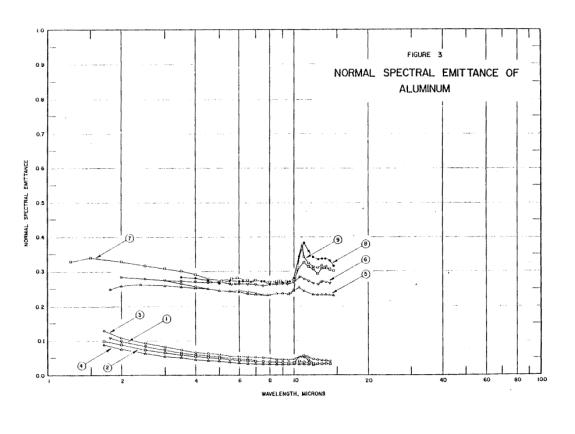
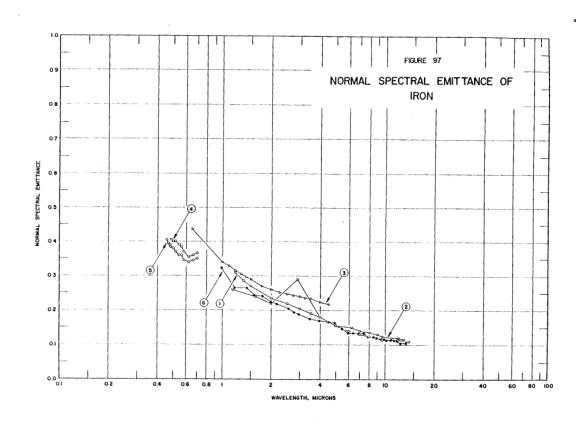
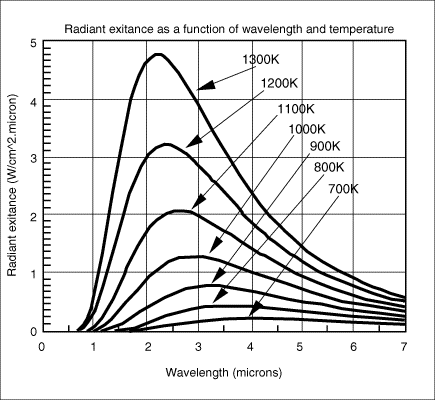
There were sixty tons of aluminum alloy right above that spot. We can only see a couple of tons of it, so you were right - the rest flowed elsewhere. One can theorize that the liquid was released when attachments holding that distorted piece of floor broke.Aluminium would be orange in colour at 1000 degrees but as the metal is already a liquid and is a flowing liquid it if were aluminium it would have melted at a temperature lower than 1000c and have already flown away from the heat source.
You have fallen victim to misunderstanding the scale effect. A small amount of material will lose heat on a cubic scale: twice the amount will cool eight times more slowly. Falling out of the tower we see hundreds of pounds of material falling per second.Only aluminium contained to a crucible has managed to keep its orange yellow colour as experiments have shown, as soon as it is poured out onto other metals it loses it temperature and reverts to silver in colour instantly.
And you know that - how?Liquid Iron/Eutectic mixture doesnt display this effect.
It cannot be thermite/thermate because, as I have already told you, it would have fallen inside the building due to its formation temperature of 2,500 deg C.Where is a source for sulphur, gypsum or thermate ? I think the gypsum has already been debunked so Thermate is the main suspect now.
At your service, sir.Find me one metallurgist who thinks this is aluminium
Actually, I don't think it's aluminum. I think it's a high-strength copper/aluminum alloy...
Last edited: