You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Molten and Glowing Metal
- Thread starter Boston
- Start date
Ron J
Active Member
har har, I've heated with wood and coal for decades, converted to veggie oil heat just a few years ago, hell I run my truck on used motor oil and even my car on bio diesel. I've designed and build my own systems since I've first owned the house. Not only that I've two forges at the shop, ones large enough to make pots in, the other for smaller stuff. Anvils hammers tongs the works. Suggesting I don't have the knowledge or experience to know how to get metal up to temp is just ridiculous.
again, smoldering for weeks and burning at 2000+ degrees F for up to two months after the event are completely different things.
the only way to get a hydrocarbon fuel to burn at those temps is to force the reaction with either fuel pressure, as in a jet engine or forced air, as in a bellows type system. So where is the evidence of either a pressurized volume of fuel being constantly and consistently fed into these multiple areas of superheated material. Or, where is the evidence of jets of fresh air/oxygen being forced both into and then exhausting from the rubble pile.
Even if you tried the chimney effect argument, you'd still need evidence of an exhaust jet, which no one saw at the WTC site. Instead we have only reports of a steaming rubble pile and pictures of red hot pockets being uncovered, that are not in association with superheated jets of exhaust air, which by the way would have been extremely hazardous and most certainly would have been reported.
again this seems like basic denial, can anyone show pockets of red hot and near molten piles of steel being removed from any other demolition site, controlled or otherwise, weeks after the event ?
Barring that evidence, I don't see much but a basic physiological need to believe in the altruistic nature of man, stepping to the forefront and protecting ones sense of welbeing from the realities of life.
Sometimes reality is just a little to harsh.
Thermite on the other hand provides its own oxygen and does result in temps like what we see at WTC 7, enough thermite later and those temps when insulated by tons of rubble, will persist for quite some time.
Enough thermite later- that is the problem. That thermite burns at 2000 degrees and provides its own oxygen, isn't in itself enough.
If someone was using thermite to cut beams, they would be using a finite amount for the singular purpose of cutting the beam, not an amount to cause a substantial piece of steel to glow in a rubble pile.
"again this seems like basic denial, can anyone show pockets of red hot and near molten piles of steel being removed from any other demolition site, controlled or otherwise, weeks after the
event ?"
I don't know how you can compare the WTC site to any other. Two 110 story buildings that were on fire and a 47 story building that was on fire when they collapsed. In controlled demolitions, buildings are not on fire and are empty shells that have nothing to burn.
Ron J
Active Member
Just the same, is the answer.
The very point of my argument is that in several places in the wreckage of 811 there would exist pieces of steel which have been made to hammer ground zero multiple times, on each occasion of which the temperature would have been made to increase. It is indeed the only mechanism by which the temperatures down there could have been increased.
This, as I have interminably stated previously, is by the process of elastic kinetic energy transfer.
Just the same, is the answer.
I can understand the concept that if i hold two flaming matches in front of me, that both will be the same temperature. I can understand heat transference through friction. Sliding two beams against each other would produce heat. I can't say i have thought about two pieces of metal crashing into each other and causing heat.
The exterior columns peeled away and fell haphazardly outside the footprint. I am presuming you are referring more to the interior columns, which would have fallen within the footprint and a narrower field, onto the beams and columns below them, as the Tower disintegrated.
The amount of heat generated would be enough to result in what appears to be a piece of glowing metal in the jaws of the excavator?
MikeC
Closed Account
I'm still confused about where this idea that the fire had to be produced by "hydrocarbon fuel" comes from.
As has been mentioned hot iron will react exothermically with steam to produce hydrogen - and then if that hydrogen doesn't burn itself it will probably reform with oxygen to form water (also exothermic), which would then be available to react with hot iron again!
Plus any gypsum in drywall being heated will generate water (steam) and possibly sulphur - which may then combine exothermically with iron also!
plus of course all the water being poured onto the hot ruins would become possible fuel!!
None of these are "combustion" as such - but they are all potential chemical reactions in the pile of rubble, and they are all exothermic!
As has been mentioned hot iron will react exothermically with steam to produce hydrogen - and then if that hydrogen doesn't burn itself it will probably reform with oxygen to form water (also exothermic), which would then be available to react with hot iron again!
Plus any gypsum in drywall being heated will generate water (steam) and possibly sulphur - which may then combine exothermically with iron also!
plus of course all the water being poured onto the hot ruins would become possible fuel!!
None of these are "combustion" as such - but they are all potential chemical reactions in the pile of rubble, and they are all exothermic!
Jazzy
Closed Account
The oxygen level deep down in the rubble was probably ZERO. It would have been removed by all the reducing materials present. "Air gaps" would have been "nitrogen gaps" if anyone had measured them. Any oxygen present would have been a constituent derived from the water of firemen's hoses. At the temperature of the deeply-buried wreckage this oxygen would have been mildly available. By that, I mean an endothermic reaction.I'm not sure it is the only mechanism by which temperature could have increased - a fire in the bottom of a pile of rubbish will increase temperature - and if the heat cannot escape surely it might do so to temperatures well above the normal temperature of combustion??
The only mechanism to repeatedly heat the ground zero steel was impact from above. For a brief while that was all there was. Its effects were successive. This is the only process that gives a temperature gain over time. There is no other.
Wake up, MikeC. Wake up Mick, for that matter.
Last edited:
Jazzy
Closed Account
Not because heat was handed on (it's too slow a process) but because kinetic energy is easily handed on by steel, which is a very elastic material. This kinetic energy (from pieces of steel hitting the top of the wreckage) is handed on (by point-to-point contact) all the way to the steel in contact with ground zero. This was by no means an efficient process, but it was successive - every impact served to raise the temperature of the steel involved.The amount of heat generated would be enough to result in what appears to be a piece of glowing metal in the jaws of the excavator?
MikeC
Closed Account
The only mechanism to repeatedly heat the ground zero steel was impact from above. For a brief while that was all there was. Its effects were successive. This is the only process that gives a temperature gain over time. There is no other.
If that was all there was "for a brief time" then how did it persist for weeks? And why can't some fairly obvious chemical reactions that are no combustion have been part of the longer term heat sources?
Jazzy
Closed Account
The heat persisted for weeks because it couldn't escape through the insulation materials in the wreckage. They were lighter and more friable than the steel, and would have been mainly above the steel for those reasons.If that was all there was "for a brief time" then how did it persist for weeks? And why can't some fairly obvious chemical reactions that are no combustion have been part of the longer term heat sources?
The hot iron/water oxidation processes are AFAIK endothermic and would have subtracted heat energy as they made hydrogen gas. I'm not familiar with the sulfur/iron reactions and cannot say anything about which way the heat has to move in that case. If there are other reactions (there may be) then I just don't know what they are.
MikeC
Closed Account
Yes the insulation effect is my point.
Steam + iron is exothermic - as is the creation of rust at room temperatures - but it is so slow normally that the energy released is not noticeable. However a simple experiment can provide evidence of it - using vinegar and steel wool to show rusting is an exothermic reaction.
some figures for industrial use of the process are given in this paper - I'm too long out of school to translate the specific quantities involved sorry - but see page 4 of it for a table of reaction energies.
Fe + S is also exothermic when the elements are heated together. And the iron sulphide that results is itself highly reactive in the presence of free oxygen - such as when dug up or air passages are created.
Steam + iron is exothermic - as is the creation of rust at room temperatures - but it is so slow normally that the energy released is not noticeable. However a simple experiment can provide evidence of it - using vinegar and steel wool to show rusting is an exothermic reaction.
some figures for industrial use of the process are given in this paper - I'm too long out of school to translate the specific quantities involved sorry - but see page 4 of it for a table of reaction energies.
Fe + S is also exothermic when the elements are heated together. And the iron sulphide that results is itself highly reactive in the presence of free oxygen - such as when dug up or air passages are created.
Last edited:
Jazzy
Closed Account
Happy to stand corrected.Steam + iron is exothermic
Boston
Active Member
Indeed, the distinction is not semantics. It's the difference between temperature and heat. You can't convert a heat value (energy) into a temperature value (average energy density) without also knowing the mass and the specific heat capacity.
Energy = mass * specific heat capacity * temperature. (in Joules and Kelvin)
This might be confusing, but illustrates one of the problems with debunking. Very often people run up against the limits of their knowledge, and then instead of extending their knowledge (which is very hard), they fall back either on common sense, or upon a rather simplified and distorted science. (Nothing personal Boston, just a general observation).
No worries. I just thought the distinction awfully slim to warrant the distraction away from the simple facts. Photographic evidence does seem to support the concept that super heated metals ( and yes most likely steel ) were present in the WTC rubble pile. I would be curious as to exactly what your thinking is extending my education in this regard ? Are you suggesting that there is some misunderstanding between directly convertible and convertible ?
But I did like the hypocrisy Jazzy
Lets just look at this definition again
Celsius heat unit (Chu)
a unit of heat energy equal to the energy required to raise the temperature of one pound of water by 1°C at standard atmospheric pressure. 1 Chu is equal to exactly 1.8 Btu, approximately 453.59 IT calories (see above), or 1.8991 kilojoules. The unit is also called the centigrade heat unit.
Now wasn't it you who said there was enough energy to in the fall of the towers to melt 840 tons of steel ? Sounds like you converted it just fine, what your really arguing is "direct conversion"
Pretty specious argument if you ask me.
But if it makes my detractors feel better. Nope, I did not identify the distinction, ( course neither did you guys when you suggested how much energy it takes to melt steel ) so now what ? Cause if your hoping I'm not willing to agree there is one, then your going to have to try again.
Next
Boston
Active Member
Jazzy, you failed to make the appropriate changes in the model to actually depict what went on in the rubble pile. So why present it again ? Have we not concluded that simply stating the same thing over and over does not make it any more valid ?
Your model represents a perfectly symmetrical system, one that cannot exist in a random collapse.
Your model represents a perfectly symmetrical system, one that cannot exist in a random collapse.
Celsius heat unit (Chu)
a unit of heat energy equal to the energy required to raise the temperature of one pound of water by 1°C at standard atmospheric pressure. 1 Chu is equal to exactly 1.8 Btu, approximately 453.59 IT calories (see above), or 1.8991 kilojoules. The unit is also called the centigrade heat unit.
Now wasn't it you who said there was enough energy to in the fall of the towers to melt 840 tons of steel ? Sounds like you converted it just fine, what your really arguing is "direct conversion"
Pretty specious argument if you ask me.
But if it makes my detractors feel better. Nope, I did not identify the distinction, ( course neither did you guys when you suggested how much energy it takes to melt steel ) so now what ? Cause if your hoping I'm not willing to agree there is one, then your going to have to try again.
Next
Sorry Boston, but I'm afraid you still seem to miss the distinction. What exactly are you saying is a "specious argument?" Jazzy calculated the potential energy of the tower, and then divided it by the energy required to melt a ton of steel. That gives you the number of tons of steel that amount of energy could melt (assuming perfect efficiency). It's a perfectly reasonable conversion
Boston
Active Member
I'm still confused about where this idea that the fire had to be produced by "hydrocarbon fuel" comes from.
As has been mentioned hot iron will react exothermically with steam to produce hydrogen - and then if that hydrogen doesn't burn itself it will probably reform with oxygen to form water (also exothermic), which would then be available to react with hot iron again!
Plus any gypsum in drywall being heated will generate water (steam) and possibly sulphur - which may then combine exothermically with iron also!
plus of course all the water being poured onto the hot ruins would become possible fuel!!
None of these are "combustion" as such - but they are all potential chemical reactions in the pile of rubble, and they are all exothermic!
You don't have a containment device to pressurize the steam so it holds anything even remotely like the temps required. Steam expands, check Charles law, or maybe its in Boyles law. But its pretty darn well established your not going to get steam up to 400°C or whatever that was, outside of a pressure vessel
Cheers
B
Boston
Active Member
Sorry Boston, but I'm afraid you still seem to miss the distinction. What exactly are you saying is a "specious argument?" Jazzy calculated the potential energy of the tower, and then divided it by the energy required to melt a ton of steel. That gives you the number of tons of steel that amount of energy could melt (assuming perfect efficiency). It's a perfectly reasonable conversion
Well that would be the error right there, assuming I missed the distinction in the first place. Your assuming I'm unaware of the difference between a CHU and C. Even tho I posted the definition to show that although its not directly convertable its still highly related ?
Your still arguing directly convertible and convertible, yes, a pretty specious argument since both forms of conversions have been used within multiple conversations.
Boston
Active Member
Enough thermite later- that is the problem. That thermite burns at 2000 degrees and provides its own oxygen, isn't in itself enough.
If someone was using thermite to cut beams, they would be using a finite amount for the singular purpose of cutting the beam, not an amount to cause a substantial piece of steel to glow in a rubble pile.
"again this seems like basic denial, can anyone show pockets of red hot and near molten piles of steel being removed from any other demolition site, controlled or otherwise, weeks after the
event ?"
I don't know how you can compare the WTC site to any other. Two 110 story buildings that were on fire and a 47 story building that was on fire when they collapsed. In controlled demolitions, buildings are not on fire and are empty shells that have nothing to burn.
ah but who says it must be thermite. I'm just suggesting one possibility. We don't actually know what caused, what looks like steel beams to look like they are glowing in extremely hot radiative colors
Boston
Active Member
Uh huh, there 's about 2000 J per 1°C
Are you seriously going to argue this simple conversion doesn't exist
Conversion formula
The formula for converting a specific value from joules to Celsius heat units (IT) is:
X joules * cf = Y Celsius heat units (IT)
where
X = the specific value to be converted (in joules)
cf = the conversion factor from joules to Celsius heat units (IT)
Y = the result (in Celsius heat units (IT))
Sample calculation: let's suppose that you have a value of energy of 459 joules and want to express it in Celsius heat units (IT).
459 J = (459 x 5.265650668407317E-4) CHU
459 J = 0.24169336567989588 CHU
If this is what you guys are on about, yes its a pretty specious argument
J to C is not directly compatible but since
For water only multiply by 4.184 since 1 calorie = 4.184 joules, and 1 calorie is the amount of heat needed to raise 1gram of water 1 degree Celsius.
From this we could determine, the number of calories within x amount of water heated to x degree. We could also determine the temp in C if we knew how many J were imparted to x amount of water.
Pretending someone with an opposing argument doesn't understand this, is IMHO a very specious argument.
Course it wasn't a problem when someone else who just happened to agree with you did the exact same thing. But hey,
Last edited:
Pete Tar
Senior Member
Is 'super-heated metal' a technical term? What does it mean to super-heat something?...Photographic evidence does seem to support the concept that super heated metals ( and yes most likely steel ) were present in the WTC rubble pile....
Well that would be the error right there, assuming I missed the distinction in the first place. Your assuming I'm unaware of the difference between a CHU and C. Even tho I posted the definition to show that although its not directly convertable its still highly related ?
Your still arguing directly convertible and convertible, yes, a pretty specious argument since both forms of conversions have been used within multiple conversations.
That's not what I'm arguing. I'm just saying that Jazzy's original point about you being incorrect in converting Joules to Celsius was correct. And your claim that Jazzy did the same thing is wrong.
You don't have a containment device to pressurize the steam so it holds anything even remotely like the temps required. Steam expands, check Charles law, or maybe its in Boyles law. But its pretty darn well established your not going to get steam up to 400°C or whatever that was, outside of a pressure vessel
Cheers
B
How does air get that hot in a fire then?
Steam is just a gas, like air. If it's in a fire, then it will get hot.
Jazzy
Closed Account
But WE do. It's YOU that doesn't.We don't actually know what caused, what looks like steel beams to look like they are glowing in extremely hot radiative colors
Try grasping the other end of the stick. It's the right end.
Steel cannot be "superheated" in the same way that steam can. Calculating the heat involved to raise the temperature of iron from ambient to melting point is not cut-and-dried because the specific heat of the steel alters subtly as the steel passes through its crystalline phases, which are themselves dependent on the carbon content of the steel. I used an average value...
Nevertheless, as Mick says, it is a perfectly reasonable calculation to make, and it bears little relationship to what you were proposing.
It isn't reasonable either to insist that no kinetic energy remained when the steel hit the wreckage, or that when it did so it never hit other pieces of steel.
This allows for successive impact events to occur at the base of the wreckage which would account for very hot steel deep down in the wreckage - an example of a very massive beam bent in two I provided. Such steel cannot bend that way without cracking from cold.
Therefore one may reasonably infer it was already very hot.
Somewhere in the mists of time I forgot which way the heat flowed in the iron/water reaction, and I've never considered the sulfur/iron heat flow, but those two reactions would certainly have helped to maintain the temperatures down there.
Thanks for the hypocrisy charge. It's difficult to make that one stick, I think, for I have no love for the actions of that administration, and my only motivation is to clear this log-jam, the better to prosecute those responsible for the failure to prevent the disaster in the first place. Your actions, and those of people like you, interfere with this completely.
Last edited:
Boston
Active Member
Is 'super-heated metal' a technical term? What does it mean to super-heat something?
super heated is a term typically applied to a substance brought to a temp beyond which it typically phase changes.
In this case I'm using it in reference to a metal which appears to be heated beyond the max temp of its environment. IE the "official" story is that basic office fires somehow caused the metal to heat past its point of stability given the structural loading. The point is that although multiple forms of evidence exist supporting claims that temps of at least 1000°C and possibly upwards of 1200°C, existed, there is that sticky little issue of when office combustibles in a perfect environment ( one that certainly cannot exist in each of the three rubble piles being considered ) do not burn at temps higher than about 825°C and seldom higher than about 4~500°C; we cannot account for this excessive heating.
Special chambers/pressures are used to heat substances beyond there typical limits, in this case we have a limit thats been exceeded, without those special considerations, ergo my use of the term, super heated.
Its kinda an inside joke to imply that some manufactured event must have occurred to create the excessive temps observed
Last edited:
Boston
Active Member
That's not what I'm arguing. I'm just saying that Jazzy's original point about you being incorrect in converting Joules to Celsius was correct. And your claim that Jazzy did the same thing is wrong.
And again your not accepting the fact that Jazzy failed to identify CHU vs C which is what all this BS started over. Matter of fact I don't recall anyone ever making that distinction even when discussing volume dependent calculations. Your applying a blatant double standard in a desperate attempt to find fault when in fact, if there is a flaw, its one thats existed within the conversation from the go, and one thats been offered by pretty much every poster involved. But hey, if it makes you feel better to think someone who disagrees with you has just reached there level of incompetence, rather than simply not confusing the issues with minutia, then go right ahead.
A very specious argument indeed, when such double standards are applied. Maybe you can quote each time anyone specifically used the CHU designation when referencing the energy needed to heat the volume of materia to x degrees and each time they didn't. Then maybe you can get back to me on my failure to designate between the two, myself. Until then, this is just another pathetic attempt to discredit an alternative view.
There is bloody yellow/white glowing hot metal being pulled from that rubble pile. Its color alone indicates a temp somewhere in the 1100°C range. The mechanism needed to produce that temp, is as of yet, undefined. And no, simple office fires are not hot enough, I provided a detailed analysis of this earlier.
Boston
Active Member
But WE do. It's YOU that doesn't.
I love the attempt to isolate my opinion. Classic psychological BS. But I must admit I'm getting pretty board with this constant reiteration of the failed Newtons swing argument. The facts remain as stated. The kinetic energy could not have been transferred/concentrated in any specific area. Kinetic energy is unique to each individual component. Each component releases that energy in an asymmetrical fashion due to the random nature of disassociation, and its interaction with other elements of the collapse, unless of course, that intrinsic random nature was somehow altered through some "control" that led to a symmetrical failure. In which case you'd now have a controlled disassociation/demolition, but still have the relatively random development of the rubble pile. In which case your right back to square one and your Newtons swing model is not applicable because you don't have the perfect alignments needed nor do you have the perfect contact, what you have is about 300,000 tons of stuff thats not steel screwing up the transfer process and a pile of randomly placed twisted and glowing yellow/white hot steel that reacts in plastic deformation, thus distributing the kinetic energy throughout the rubble, rather than concentrating it in any one location.
Boston
Active Member
How does air get that hot in a fire then?
Steam is just a gas, like air. If it's in a fire, then it will get hot.
Um, steam expands
Its really pretty simple
at normal atmospheric pressure water turns to steam at 100°C if you change that pressure you alter its phase change point. Without that alteration in pressure, your going to get steam at 100°C. Period. Increase the pressure, and you increase the boiling temperature. Something else to consider is that steam expands to about 1600 times the volume of the water it converted from, which brings the steam some significant distance from the source of heat as well as begins the process of shedding that heat.
the steam did it hypothesis is long dead. Yup there is Swiss cheese looking metal coming up from the bottom ? of the rubble, we actually don't have any clue as to where any of few samples that were saved came from. But the dramatic failure to properly investigate "all" elements of this event preclude any real conclusions from being drawn as to the origins of the required heat source.
Last edited:
Jazzy
Closed Account
I didn't bother, Boston, because I knew already that you weren't on the right track. There is no point in trying to make sense of something that begins as nonsense. I'm just being efficient, not careless.And again your not accepting the fact that Jazzy failed to identify CHU vs C which is what all this BS started over.
There is no need to if one begins one's calculations correctly, keeping one's units (mass, length, time) in balance both sides of the equation. As soon as one sees this hasn't been done, there is no point proceeding with any further analysis.Matter of fact I don't recall anyone ever making that distinction even when discussing volume dependent calculations.
Or maybe it's YOU.Your applying a blatant double standard in a desperate attempt to find fault
Metric units are very much easier to use.Maybe you can quote each time anyone specifically used the CHU designation
That lacks, er, truth. I have defined them, and you have yet to find fault either with my maths or with my reasoning.a temp somewhere in the 1100°C range. The mechanism needed to produce that temp, is as of yet, undefined.
I think we all accept that.And no, simple office fires are not hot enough, I provided a detailed analysis of this earlier.
Earlier I said: "an example of a very massive beam bent in two I provided*. Such steel cannot bend that way without cracking from cold. Therefore one may reasonably infer it was already very hot".

Here is the example once again. It is a column, not a beam - apologies. If you look at its section you can see that it is from lower down the tower than where the fire was burning - at least four hundred feet lower. So it wasn't heated by the fire. It is also obvious that thermite took no part in its heating.
It is also obvious that it was bent after it was heated. So the order is a) it fell, b) it was heated, and c) it then bent. This is still consistent with my explanation.
Here is a discussion on how steel has to be already hot in order to bend without cracking:
* It isn't the only example.
Last edited by a moderator:
Boston
Active Member
I didn't bother, Boston,
ahahahahahahahaha

well then
I guess I didn't bother either
because I knew already that you weren't on the right track. There is no point in trying to make sense of something that begins as nonsense. I'm just being efficient, not careless.
I liked your reasoning sooooo much, I opted for the same excuse
Last edited by a moderator:
Boston
Active Member
It is also obvious that it was bent after it was heated. So the order is a) it fell, b) it was heated, and c) it then bent. This is still consistent with my explanation.
* It isn't the only example.
. We can know two things from this photo, that the steel was bent and that the steel wasn't likely bent cold. We do not know when it was bent, either in the rubble pile or as pieces fell on it in its original position. If it was bent in the rubble pile, then its likely an example of that plastic deformation I was talking about. The energy it took to bend this piece did not transfer downward through the pile, it was expended right then and there bending this whopping huge beam/column ( I'd want to see blue prints before subjecting myself to another perfectly assenhine semantic argument ) . Either way, its a great example of how the energies dissipated, randomly, little by little, rather than concentrated in any single location. Unless that is you can show a Newtons swing made of sponges and explain just why its not working
Last edited:
Jazzy
Closed Account
That my units and dimensions weren't correct?I opted for the same excuse
But we DO. In its original position it was cold, and it would have cracked.We do not know when it was bent, either in the rubble pile or as pieces fell on it in its original position.
Actually, I can.Unless that is you can show a Newtons swing made of sponges and explain just why its not working
Concrete-filled steel globes don't crack it. You should sympathize.
Concrete isn't elastic...
Last edited:
Boston
Active Member
But we DO. In its original position it was cold, and it would have cracked.
Ah but your assuming when it was heated, when in fact we don't know when it was heated or to what degree it was heated, nor do we know when it was bent. So again the assumption that it must have this or must have that is flawed
Actually, I can.
Opps, progress, wasn't expecting that, soooooooo why is it a Newtons swing made of sponges doesn't work then ???????
Concrete-filled steel globes don't crack it. You should sympathize.
Concrete isn't elastic...
Sure but we don't have any concrete filled steel globeswhat we have is a 200,000 ton pile of twisted broken steel separated by about 300,000 tons each tower of pulverized and powdered concrete, which is highly elastic

Ever shot a bullet into sand ? Doesn't go to far does it ?
Um, steam expandsair is not steam
you might want to check Charles law, or maybe its in Boyles law, its been a while, but expanding gasses release heat, without a containment device, you can only get steam so hot.
Its really pretty simple
at normal atmospheric pressure water turns to steam at 100°C if you change that pressure you alter its phase change point. Without that alteration in pressure, your going to get steam at 100°C. Period. Increase the pressure, and you increase the boiling temperature. Something else to consider is that steam expands to about 1600 times the volume of the water it converted from, which brings the steam some significant distance from the source of heat as well as begins the process of shedding that heat.
the steam did it hypothesis is long dead. Yup there is Swiss cheese looking metal coming up from the bottom ? of the rubble, we actually don't have any clue as to where any of few samples that were saved came from. But the dramatic failure to properly investigate "all" elements of this event preclude any real conclusions from being drawn as to the origins of the required heat source.
Air is a gas. If air can get that hot, so can steam.
Jazzy
Closed Account
I'm assuming nothing. In its original position it was cold, and it would have cracked.Ah but your assuming when it was heated, when in fact we don't know when it was heated or to what degree it was heated, nor do we know when it was bent. So again the assumption that it must have this or must have that is flawed
Because sponge isn't very dense, isn't very elastic (though more so than concrete) and suffers from internal and external air resistance.why is it a Newtons swing made of sponges doesn't work then?
Wow."Concrete isn't elastic" - Sure
More.what we have is a 200,000 ton pile of twisted broken steel
Less, and I thought we had established that concrete isn't elastic.separated by about 300,000 tons each tower of pulverized and powdered concrete, which is highly elastic
Yes.Ever shot a bullet into sand?
It does when it's made of steel. A copper bullet tends lose its shape, and it is that which stops it. I've seen bullets leave the sand almost as fast as they entered it. They kept their shape... ...I think...Doesn't go too far does it?
Massive steel columns and beams are three times denser than concrete rubble, and will part it with ease and with little deformation when they arrive at 120 mph. When they hit steel they will stop, having cheerfully handed on their kinetic energy. It's Newton's Cradle - it's snooker - it's billiards.
Jazzy
Closed Account
You don't really know it, but that is, er, true.you can only get steam so hot.
Once it gets up to the temperature of a hydrogen flame burning in oxygen, it's essentially hot hydrogen with hot oxygen. It's only when it falls beneath that flame temperature that it decides to become steam. But this burning happens at ambient pressure, so what you keep maintaining is, er, false.
No, it's YOU.Its really pretty simple
Any old railway engineer would tell you that such corrosion is caused by steam at the temperatures we've been discussing. It's about time you stopped pretending knowledge while demonstrating ignorance.the steam did it hypothesis is long dead. Yup there is Swiss cheese looking metal coming up from the bottom of the rubble, we actually don't have any clue as to where any of few samples that were saved came from.
Not in a modern technological world, it shouldn't. Just in your case, it does.But the dramatic failure to properly investigate "all" elements of this event preclude any real conclusions from being drawn as to the origins of the required heat source.
Boston
Active Member
wrong on all counts
Air doesn't go threw a phase change, so its initial state is not one of rapid expansion, thus the majority moving away from the heat source. Yup once expanded it can get hotter, except both you guys are forgetting something very basic. The pressure is directly related to the temp, ergo as steam gets hotter, it continues to expand, continues to diffuse into atmosphere and continues to release heat energy to atmosphere. There was no mechanism to to contain it in the rubble pile. So as it heated beyond its phase change point, it expanded, releasing heat and removing itself from the heat source. Sure any gas can be heated, but without a containment vessel, its molecular density reduces proportionally. You guys are grasping at straws again.
Steam didn't cause the WTC metal to glow indicating temps much much higher than what was possible given the fuels available.
indicating temps much much higher than what was possible given the fuels available.
and its wrong, try playing any of those games on a table covered in a few centimeters of concrete dust and get back to us on how well it worked I think its high time you admit you have no idea what your talking about, this theory is long dead and buried and your continued insistence only embarrassed yourself. Concrete dust will act just like straight up sand and absorb the vast majority of the energy involved. Your failure to understand that simple reality is glaring at this point
I think its high time you admit you have no idea what your talking about, this theory is long dead and buried and your continued insistence only embarrassed yourself. Concrete dust will act just like straight up sand and absorb the vast majority of the energy involved. Your failure to understand that simple reality is glaring at this point
Oh and your making assumptions again
Denial of the obvious, you don't know it was cold in its original position, or where you up there taking measurements just before the collapse you just don't know how it may have been heated at the time of collapse, or when. Your also assuming it would have cracked. Funny but for as bad a picture as you've presented, it certainly looks like the back side of that "column" ? ( oh lord here it comes ) cracked just fine. Your example, poor as it is, fails again to support your theory of assumptions.
you just don't know how it may have been heated at the time of collapse, or when. Your also assuming it would have cracked. Funny but for as bad a picture as you've presented, it certainly looks like the back side of that "column" ? ( oh lord here it comes ) cracked just fine. Your example, poor as it is, fails again to support your theory of assumptions.
I gotta admit, I think the fascination for me at least with these kinda threads is watching people who claim all kinds of higher education, making wildly implausible suggestions, and presenting absolutely ludicrous hypothesis, pretending they have some basis in chemistry or physics, rather than just admit they don't have a clue.
The only large objects reported to have survived within the rubble piles were steel components. I've yet to see any of you provide the requested pictures of anything like a large chunk of office furniture or any other large chunk of combustible that survived the demolition. Yet when a large piece of material, complete with geometric shape and glowing hot, without flame or smoke, dripping slag and being held up in the teeth of a 40 ton backhoe, after being wrenched from a pile of tons of "other" steel beams and concrete, without disintegrating instantly, or bursting into flame; is shown, you guys just can't admit the obvious.

and this is not the only picture of metal glowing hot in the 1100°C range. Nor is it the most recognizable piece in the photographic evidence

Ten days later its pretty obvious there's a large piece of C channel embedded in a freshly excavated ( the excavator is just behind the glowing hot debris ) chunk of rubble.
While you might argue its not steel, if the picture is in fact from one of the two tower sites rather than the WTC 7 site, then there was about 120 tons of aluminum airplane material each tower, although none in WTC 7. Which means there's a 0.000875 chance this piece is aluminum so as we all can see, the odds against the "its not steel" argument are formidable.
so as we all can see, the odds against the "its not steel" argument are formidable.
In the end, its more than obvious we have multiple examples of super heated steel which could not have been caused by office fires. The steam did it theory is long dead, you can't get a much steam up to the 400°C without a huge amount of containment pressure. Something like 4363 PSI and even if you did have a minor amount of superheated steam available, you'd still need to find a heat source to get it that hot.
heated steel which could not have been caused by office fires. The steam did it theory is long dead, you can't get a much steam up to the 400°C without a huge amount of containment pressure. Something like 4363 PSI and even if you did have a minor amount of superheated steam available, you'd still need to find a heat source to get it that hot.
I wonder if our readers are noticing that amid the torrent of derogatory comments my arguments remain focused on actual photographic evidence and do not depend on poorly constructed hypothesis and supposition, with a whole bunch of assumptions thrown in.
For instance
How about these samples of molten concrete noted at the 9/11 museum.

as for that ridiculous Newtons swing argument
Air doesn't go threw a phase change, so its initial state is not one of rapid expansion, thus the majority moving away from the heat source. Yup once expanded it can get hotter, except both you guys are forgetting something very basic. The pressure is directly related to the temp, ergo as steam gets hotter, it continues to expand, continues to diffuse into atmosphere and continues to release heat energy to atmosphere. There was no mechanism to to contain it in the rubble pile. So as it heated beyond its phase change point, it expanded, releasing heat and removing itself from the heat source. Sure any gas can be heated, but without a containment vessel, its molecular density reduces proportionally. You guys are grasping at straws again.
Steam didn't cause the WTC metal to glow
Massive steel columns and beams are three times denser than concrete rubble, and will part it with ease and with little deformation when they arrive at 120 mph. When they hit steel they will stop, having cheerfully handed on their kinetic energy. It's Newton's Cradle - it's snooker - it's billiards. and its so wrong its downright humorous
and its wrong, try playing any of those games on a table covered in a few centimeters of concrete dust and get back to us on how well it worked
Oh and your making assumptions again
I'm assuming nothing. In its original position it was cold, and it would have cracked.
Denial of the obvious, you don't know it was cold in its original position, or where you up there taking measurements just before the collapse
I gotta admit, I think the fascination for me at least with these kinda threads is watching people who claim all kinds of higher education, making wildly implausible suggestions, and presenting absolutely ludicrous hypothesis, pretending they have some basis in chemistry or physics, rather than just admit they don't have a clue.
The only large objects reported to have survived within the rubble piles were steel components. I've yet to see any of you provide the requested pictures of anything like a large chunk of office furniture or any other large chunk of combustible that survived the demolition. Yet when a large piece of material, complete with geometric shape and glowing hot, without flame or smoke, dripping slag and being held up in the teeth of a 40 ton backhoe, after being wrenched from a pile of tons of "other" steel beams and concrete, without disintegrating instantly, or bursting into flame; is shown, you guys just can't admit the obvious.

and this is not the only picture of metal glowing hot in the 1100°C range. Nor is it the most recognizable piece in the photographic evidence

Ten days later its pretty obvious there's a large piece of C channel embedded in a freshly excavated ( the excavator is just behind the glowing hot debris ) chunk of rubble.
While you might argue its not steel, if the picture is in fact from one of the two tower sites rather than the WTC 7 site, then there was about 120 tons of aluminum airplane material each tower, although none in WTC 7. Which means there's a 0.000875 chance this piece is aluminum
In the end, its more than obvious we have multiple examples of super
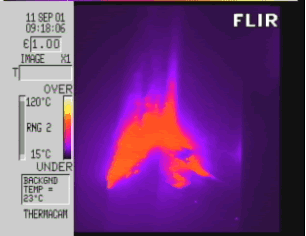
5) The thermographic image of the South Tower taken by Carol Ciemiengo 15 minutes after it was hit by Flight 175, which shows temperatures of around a mere 90 to 100 degrees Celsius! But, only 40 minutes later, the fires were supposedly hot enough to not only melt "aluminium" but make it glow orange-yellow.

I wonder if our readers are noticing that amid the torrent of derogatory comments my arguments remain focused on actual photographic evidence and do not depend on poorly constructed hypothesis and supposition, with a whole bunch of assumptions thrown in.
For instance
How about these samples of molten concrete noted at the 9/11 museum.

as for that ridiculous Newtons swing argument
Last edited by a moderator:
wrong on all counts
Air doesn't go threw a phase change, so its initial state is not one of rapid expansion, thus the majority moving away from the heat source. Yup once expanded it can get hotter, except both you guys are forgetting something very basic. The pressure is directly related to the temp, ergo as steam gets hotter, it continues to expand, continues to diffuse into atmosphere and continues to release heat energy to atmosphere. There was no mechanism to to contain it in the rubble pile. So as it heated beyond its phase change point, it expanded, releasing heat and removing itself from the heat source. Sure any gas can be heated, but without a containment vessel, its molecular density reduces proportionally. You guys are grasping at straws again.
Steam is a gas. It has already gone through the phase change. So it's not really vastly different to air.
Again, if the air near a fire can reach high temperatures, then why not steam?
I don't think it has a vast bearing on the conversation. But we need to get basic facts right.
I wonder if our readers are noticing that amid the torrent of derogatory comments my arguments remain focused on actual photographic evidence and do not depend on poorly constructed hypothesis and supposition, with a whole bunch of assumptions thrown in.
For instance
How about these samples of molten concrete noted at the 9/11 museum.

So you think the hot spots were "caused by thousands of gallons of fuel buried beneath the rubble?"
And what exactly is "molten" (or "melted") in that photo?
Last edited:
Boston
Active Member
Steam is a gas. It has already gone through the phase change. So it's not really vastly different to air.
Again, if the air near a fire can reach high temperatures, then why not steam?
I don't think it has a vast bearing on the conversation. But we need to get basic facts right.
Yes we do
Which means we need to acknowledge that in the formation of steam we have expansion, and that that expansion is synonymous with any expanding gas and will mechanically dissipate heat, not absorb it. Am I really the only one who understands the Joule Thomson effect ?
We were after all, discussing the idea that steam caused the Swiss cheese effect seen in some of the WTC steel samples, an idea that requires steam at a very high temperature, one in which saturated steam exists at a pressure of, what was it, 4363 PSI ? and you don't have a pressure vessel in the WTC rubble pile, instead we see multiple chimneys of low pressure exhaust gasses. So again there is no supporting evidence to this hypothesis. Yes there is crystallized metal, but simple heat could have done that, as is an observed fact at the site, rather than superheated steam.
Yes we do
Which means we need to acknowledge that in the formation of steam we have expansion, and that that expansion is synonymous with any expanding gas and will mechanically dissipate heat, not absorb it. Am I really the only one who understands the Joule Thomson effect ?
We were after all, discussing the idea that steam caused the Swiss cheese effect seen in some of the WTC steel samples, an idea that requires steam at a very high temperature, one in which saturated steam exists at a pressure of, what was it, 4363 PSI ? and you don't have a pressure vessel in the WTC rubble pile, instead we see multiple chimneys of low pressure exhaust gasses. So again there is no supporting evidence to this hypothesis. Yes there is crystallized metal, but simple heat could have done that, as is an observed fact at the site, rather than superheated steam.
We were discussing your claim:
your not going to get steam up to 400°C or whatever that was, outside of a pressure vessel
And I was making the point that if air could get that hot (as it does in in a fire), then steam, which is simply mixed with the air, can also get that hot.
Boston
Active Member
So you think the hot spots were "caused by thousands of gallons of fuel buried beneath the rubble?"
And what exactly is "molten" (or "melted") in that photo?
No I think the building was separated by sky lobbies specifically designed to prevent exactly that type of flammable transfer. Of the hundred or so elevators in each tower only one or two actually had a continuous shaft going to the basement. I also seem to remember there being fire doors within those shafts as well, but I'd have to look it up before making a definitive statement. Also there is the problem of oxygen, will jet fuel burn in an oxygen starved environment at its maximum temp ? I somehow doubt it, so again we have a huge disparity in temps seen vs temps available given the known fuel sources. And that assumes we are not talking about WTC 7 where there is no excuse of burning Jet fuel.
Read the information provided by the Museum by blowing up the picture and you will quickly realize they claim molten. In which case you can go argue it with them if you want.
See
http://www.google.com/imgres?imgurl=http://yankee451.com/wp-content/uploads/2012/07/Exhibit-1.png&imgrefurl=http://yankee451.com/2012/07/15/963/&usg=__U4bxKJKA7qLuhs1GR6T6kXdBUW4=&h=961&w=1469&sz=2826&hl=en&start=3&sig2=zK_ucYC28Tjtq1lKKK2MQw&zoom=1&tbnid=W61Ui6gLIheEOM:&tbnh=98&tbnw=150&ei=95EjUtSXMKfuyQH-jIC4BA&prev=/search?q=9/11+musium+molten+concrete&um=1&hl=en&gbv=2&tbm=isch&um=1&itbs=1&sa=X&ved=0CDIQrQMwAg
This site contains an image large enough to read the caption over the melted pieces
It clearly states
"fire temperatures were so intense that concrete melted like lava around anything in its path"
I got another bbq to go to but maybe if I find the time I'll note another reason the Newtons swing idea is so hilariously flawed. Its basic physics kids, no idea why its so bloody hard for some people to understand how a gravel like substance will dissipates kinetic energy.
This site contains an image large enough to read the caption over the melted pieces
It clearly states
I got another bbq to go to but maybe if I find the time I'll note another reason the Newtons swing idea is so hilariously flawed. Its basic physics kids, no idea why its so bloody hard for some people to understand how a gravel like substance will dissipates kinetic energy.External Quote:"fire temperatures were so intense that concrete melted like lava around anything in its path"
It also clearly states:
So why don't you believe what is written on one caption, but you do with another? How is helpful to post an image if you are just going to cherry pick? Maybe you should black out the areas you disagree with? Or selectively crop?External Quote:"caused by thousands of gallons of fuel buried beneath the rubble"
Latest posts
-
-
-
Synchronicity - What's your experience of it?
- Latest: Todd Feinman
-
