|
|
|
Mark R. Ferran BSEE scl
JD mcl "Iron Burns!!!" |
Below is a series of E-mails between
Mark R. Ferran BSEE scl
JD mcl and various members of the "Scholars
for 911 truth", in which he attempts to educates them on how Iron burns. Not only does it burn/oxidize, but it can burn/oxidize at low temperatures.
Sent: Tuesday, June 20, 2006 3:18 PM
Subject: WTC IRON BURNS!!!
The only likely source of the heat great enough
to actually "melt" significant quantities of
iron in the piles (or even just raise so much of
it to red-hot or to 2000F) would be chemical
energy (i.e., "combustion" of some sort).
Professor Jones assumes that all the
carbonaceous "combustible" matter in the "piles"
would have burned away long before the time that
the red-hot and molten iron was discovered
(weeks after the collapse of the WTC
towers). Perhaps it did, by weeks after the
collapse. But Professor Jones obviously does
not comprehend that the hot, red-hot and
molten IRON IS COMBUSTIBLE matter.
Here, Jones clearly missed it, when he wrote:
"At
these temperatures, steel will melt, and aluminum materials from the buildings
should continue to undergo exothermic
oxidation reactions with materials also
entrained in the molten metal pools including
metal oxides which will then keep the pools
molten and even growing for weeks despite
radiative and conductive losses. ... The
government reports admit that the building fires
were insufficient to melt steel beams -- then
where did the molten metal pools come from?"
http://www.physics.byu.edu/research/energy/htm7.html
Jones has no clue because he has conception of
Steel Burning (iron oxidation) in air.
The Truth is that: HOT STEEL WILL CONTINUE TO
UNDERGO EXOTHERMIC OXIDATION REACTIONS WHILE
EXPOSED TO AIR, CAUSING IRON TO INCREASE ITS
TEMPERATURE UNTIL IT MELTS, FORMING POOLS OF
MOLTEN IRON.
Professor Jones' comments and conjectures about
the origin of the alleged molten iron
found within the three huge piles of combustible
matter burning after the collapse of the WTC
towers, distinctly prove that Professor Jones is
oblivious of the fact that Iron Burns in air.
For perspective, I found this children's
educational webpage that further illustrates
that "Professor Jones" (among the "9-11
Scholars") is an incompetent
ignoramus because he ignores the
scientifically provable (or
disprovable) fact that Iron metal itself
burns, and that when amassed in large piles
can ignite fires (and can even melt
itself). The article discusses child-safe
experiments observing a very slow oxidation
of iron (rusting at room temperature), but
also mentions:
"Sometimes a big load of iron in a
ship can get hot. The heat can even set
other materials on fire. That’s
because the iron is rusting, which means
it is burning very, very slowly. Iron
rusts in a chemical reaction called
oxidation. That means the iron
reacts with oxygen gas from the air.
Oxidation is the chemical reaction that
occurs when anything burns in air. Like
most oxidations, rusting gives off
heat."
Beyond the scope of this child-oriented
article, it is important to understand that
general rule in chemistry that most chemical
reactions (e.g., oxidation of iron) are
accelerated by higher temperatures. This is
especially true of iron oxidation. This
means, that the hotter iron metal in contact
with oxygen is, the faster it will oxidize
(burn). For example, it is a familiar sight
at iron foundries to see hot iron rust
forming instantaneously on red-hot iron
beams. This hot rust usually falls
off spontaneously (because of the difference
in thermal expansion properties between iron
and rust). Meaning, a hot iron beam, if
combined with a large enough number of other
hot iron beams in a confined or semi
insulated pile (e.g., covered with cement
dust), will burn CONTINUOUSLY until
it consumes itself, (and thus will appear to
have been "vaporized" to those not looking
for the rust residue). It will just thin
away (and turn into rust), as illustrated by
this photo of burned and thinned I-beam
metal recovered from the rubble of the WTC
towers:
Ancient Wisdom about burning iron:
Thomas Aquinas and other theologians
remarked on the famous burning property of
Iron:
Aquinas maintains that:
The head causes an influx of sensation
and motion to all members of the body.
... [S]omeone can understand “to flow
into” (“influere”) in two ways according
to the spiritual sense and mode. One
mode as principal agent: And thus it
belongs to God alone to provide an
influx of grace in the members of the
Church. In another mode instrumentally:
And thus even the humanity of Christ is
a cause of the said influx; because as
Damascene says ... as iron
burns on account of the
fire conjoined to it, so were the
actions of the humanity of Christ on
account of the united divinity, of which
the humanity itself was an instrument.
Christ, nevertheless, according to the
two last conditions of head [governance,
influence] is able to be called head of
the angels according to human nature,
and head of both according to divine
nature; not, however, according to the
first condition [namely, sameness in
nature], unless one takes what is common
according to the nature of the genus,
according as man and angel agree in
rational nature, and further what is
common according to analogy, according
as it is common to the Son along with
all creatures to receive from the
Father, as Basil says, by reason of
which he is said to be the first-born of
all creatures, Col. 1:15. 16
http://www.unav.es/cryf/georgemaritain.html
DAMASCENUS, (lib. 3, cap. 17) wrote:
"For not according to its [the
flesh's] own operation, but by the Word
united to it, He wrought divine things,
the Word displaying through it His own
operation. For glowing iron
burns not by possessing in
a natural manner the power to burn, but
by possessing this from its union with
the fire. Therefore in itself it was
mortal, and on account of its personal
union to the Word, quickening."
http://www.iclnet.org/pub/resources/text/wittenberg/concord/web/
augsc-05.html
19th Century:
"Iron commences to 'burn' at
2500[F], while at the end of the
operation in the Bessemer process,
when the temperature reaches some
3000[F], the iron burns violently,
as demonstrated by examination of
the Bessemer flame with the spectro-
scope. (See p. 46, Vol. II.)"
Manufacturer and
builder / Volume 3, Issue 6, June 1871
Iron smiths (Blacksmiths) modern and ancient
are aware that glowing Iron Burns:
"With
bellows blowing additional air through
the fire, it can reach temperatures of
about 3,000° Fahrenheit. Iron burns at
2,800°, however, so the smith has to be
careful to not ruin his work! … The
smith's fire contains too much oxygen to
allow iron to melt; as it approaches its
melting point the iron burns instead."
Also of note: Faraday's lectures and a
demonstration of iron powder burning
incandescent in air (and more brightly
in pure oxygen):
http://www.fordham.edu/HALSALL/MOD/1859Faraday-forces.html
(" Michael
Faraday was the son of a blacksmith, and
was born at Newington Butts, near
London, September 22, 1791.")
A WWII witness in Germany recounts seeing
the "iron" of three Russian tanks
"burn" from March 9, 1945 until November 3,
1945:
http://members.tripod.com/~radde/RaddesFlight.html
(" The three
Russian tanks before Bresin still burned as
we passed by them on the morning of 11-3,
and this taught me something surprising:
iron burns.") This account
suggests that the "critical mass" of iron
metal that will sustain itself burning hot
may be quite small compared to the
huge amounts of iron debris the WTC piles.
This account of prolonged iron combustion
also supports the conclusion that the main
source of high heat in the piles of the
WTC 1, 2 and 7, weeks and months after their
collapse, was due to burning iron in these
piles. This conclusion could be readily
verified or disproved through simulation or
experimentation.
The other interesting thing about
"iron fire" (fast oxidation of iron) is
that it creates a "vacuum" of sorts that
"sucks" oxygen to itself. Ordinary
carbonaceous "fire" creates carbon
monoxide (CO) or carbon dioxide (CO2),
which are gases that can take the place
of consumed oxygen (02) gas. Carbon
monoxide production releases two
molecules of CO gas per one O2 molecule
consumed. Thus, such a carbon
fire requires a "convection" current to
remove the hot carbon mon/dioxide (out
the top) to make room for more cold
oxygen to be brought in (at the
bottom).
By contrast, an "iron fire" converts
the oxygen gas (and possibly also
nitrogen gas, but that is tangent) into
a solid (rust). Thus, the burning
iron metal effectively sucks atmospheric
oxygen INTO the pile of burning metal,
regardless of convection currents.
Convection currents are a strong
mechanism for REMOVING heat from a
fire. Of course convection currents
will also be present even in a huge iron
pile furnace, but a result of direct
conversion of oxygen gas into a solid
(rust) is that there are weaker
convection currents and that means that
the heat of combustion escapes more
slowly from the metal fire furnace than
from a carbonaceous fire furnace. Thus,
since the heat of combustion does not
leave with the combustion products, a
metal-air furnace could become
much "hotter" faster than a carbon-air
furnace of the same scale (e.g., at the
same oxygen demand level).
Theoretically, there is no limit
upon the temperature that such a large
air-metal-fire could attain. It could,
in theory, attain a temperature high
enough to not only melt iron, but also
to boil (vaporize) iron, but not at the
same location at the same time. (You
cannot maintain solid, liquid, and
gaseous iron at the same location,
because "melting" and
"vaporization" occur at greatly
different temperatures). The difficulty
with that however is that the molten
(burning) iron would tend to settle into
a pool, having a smaller surface area
(on its top surface only), thus
reducing its rate of oxidation.
It has also been suggested that Sulfur
especially from tons of decomposing
Gypsum (a Sulfur ore used in sheetrock
walls and partitions in offices and
homes) in the piles accelerated the
oxidation or melting of the iron
burning in the piles. "Sulfur is widely
distributed in nature. It is found in
many minerals and ores, e.g., iron
pyrites, galena, cinnabar, zinc blende,
gypsum..."
http://columbia.thefreedictionary.com/Sulpher
"Dust and debris deposits
associated with the September
11, 2001, terrorist attack on
the World Trade Center have left
a distinct fingerprint on the
sedimentary record in New York
Harbor, scientists have found.
Their results appear in the
January 21, 2003, issue of the
journal EOS, a publication of
the American Geophysical Union.
... The high levels of calcium,
strontium, and sulfur
concentrations found in the
near-surface sediments ..., are
consistent with presence of
gypsum as a parent material.
Gypsum is extensively used as
drywall in building
construction."
The "Sulfides" produced when
sulfur dioxide (e.g., from
decomposed Gypsum) contacts
burning iron have been
identified as an agent that
supposedly accelerated the
"deterioration" of the steel in
the burning WTC piles, on a
macromolecular level.
"A section of an A36 wide
flange beam retrieved from
the collapsed World Trade
Center Building 7 was
examined to determine
changes in the steel
microstructure ...
Rapid
deterioration of the steel
was a result of heating with
oxidation
in combination with
intergranular melting
due to the presence
of sulfur. The
formation of the eutectic
mixture of iron oxide and
iron sulfide lowers the
temperature at which liquid
can form in this steel. This
strongly suggests that the
temperatures in this region
of the steel beam approached
~1,000ºC, forming the
eutectic liquid ...."
http://www.tms.org/pubs/journals/JOM/0112/Biederman/Biederman-0112.html
In other words, Sulfur Dioxide gas
(e.g., from decomposing Gypsum
wallboard) spontaneously reacts
(combines) with iron metal (cold or
hot), turning it into iron sulfides
and iron oxides (i.e. burning the
iron). The sulfides introduced into
iron (sulfidation) by exposure of
iron to Sulfur Dioxide gas have been
used by humans (blacksmiths) for
hundreds if not thousands of years,
and have been understood in chemical
terms for centuries, but apparently,
such chemistry is not understood by
BYU Professor Jones.
"The formation of the eutectic
mixture of iron oxide and iron
sulfide lowers the temperature
at which liquid can form in this
steel. This strongly suggests
that the temperatures in this
region of the steel beam
approached ~1000°C by a
process similar to making a
“blacksmith’s weld” in a hand
forge. (Barnett, 2001)"
For hundreds of
years, Blacksmiths took advantage of
this well-known property of sulfur
dioxide by "welding" iron parts
together over fires of sulfur-rich
charcoal, which lowers the melting
point of iron at its surface.
Sulfur Dioxide gas
can be released by the burning of
ANY ORGANIC substance, including
wood, paper, flesh, fabrics, and
especially plastics (carpets),
and rubber (rubber is "vulcanized"
by adding sulfur to it). Sulfur
Dioxide gas, has a distinct impact
on the nose, and is a respiratory
irritant, because it forms sulfurous
acid when it combines with water or
moisture in the human body. Sulfur
Dioxide can be further oxidized to
form sulfuric acid (when added to
water). High
concentrations of Sulfurous fumes
emanating from the piles at Ground
Zero have been documented, and have
been identified as a probable cause
of respiratory ailments suffered by
many rescue workers and cleanup
crews.
"One of the America's top
air-quality scientists test the air
around Ground Zero and tells NBC's
Lisa Myers and the NBC Investigative
Team he was shocked to find alarming
levels of sulfuric
acid and fine particles more than
three weeks after the attack.
(MSNBC, October 29, 2003)"
http://www.asthmamoms.com/worldtradecenterarticles2003.htm
Professor Jones
demonstrates his ignorance of the
basic "Blacksmith" chemistry of
sulfidation-by-S02-from-fire with
his following oblivious or dishonest
statements: "Then
there is the rather mysterious
sulfidation of the steel reported in
this paper -- What is the origin of
this sulfur? No solid answer is
given in any of the official
reports. ... While gypsum in the
buildings is a source of sulfur, it
is highly unlikely that this sulfur
could find its way into the
structural steel in such a way as to
form a eutectic. ... Thus, we
find substantial evidence supporting
the current conjecture that some
variation of thermite (e.g., solid
aluminum powder plus Fe2O3, with
possible addition of sulfur) was
used on the steel columns of the WTC
Tower to weaken the huge steel
supports, not long before explosives
finished the demolition job."
In addition to sulfidation of cold
iron by its exposure to sulfurous
(e.g., SO2) fumes, sulfidation by an
even more direct transfer of the
sulfur and oxygen from Gypsum to
Iron might occur where Gypsum (dust)
is in direct contact with the
burning (e.g., red hot) iron.
Another's
lucid rebuttal of Professor Jones'
conjectures about the sulfidated
iron found in the burning piles of
WTC wreckage is self-published as
follows:
"The "absolutely
conclusive smoking-gun PROOF"
amounts to this: Prof. Jones
CLAIMS to have obtained a sample
of solidified spatter from
post-collapse WTC structural
steel. He takes the
sample-gatherer's word that this
is where it came from. He claims
to have determined the sample to
be sulfur-contaminated iron.
Solely from this basis he leaps
to the definite conclusion that
it's a residue of thermate
(thermite with sulfur and
potassium permanganate
additives) used to cut the
tower's columns. This is quite
the leap of inductive
reasoning. As we all know, the
debris field of the WTC was an
oven of steel-melting intensity.
All of the WTC's debris was
churned together chaotically in
this pile. Steel is basically
highly refined iron. The element
sulfur is present in abundance
in many building materials.
Drywall, for example (also known
as GYPSUM board) consists
primarily of plaster, i.e.
gypsum, i.e. hydrated calcium
SULFATE. Churn lots of steel and
gypsum together and cook them
for three weeks at temperatures
sufficient to melt both and I
would not be surprised to see
"sulfur-contaminated iron"
turning up in samples of same.
This is not to say Jones is
definitely wrong as to what
produced it, just that it's
ridiculously dishonest and
irresponsible to hype this as
"absolutely conclusive
smoking-gun PROOF" of the use of
thermate. There is at least one
other completely plausible
completely mundane possibility.
Prof. Jones focuses on the
iron/sulfur mix as a signature
of thermate, but makes no
mention of aluminum oxide, which
would also most definitely be
present and which he'd certainly
test for and mention if it were.
This is a strange omission.
Prof. Jones knows better "
For practical purposes, all this
means that a huge pile of iron
beams (e.g., mixed in with tons of
other materials initially burning) can
itself begin to burn like huge iron logs
in a pile furnace, and there is
no reason not to expect this system to
reach a temperature high enough to melt
iron. Sulfur Dioxide (SO2) gas,
released from burning organic materials,
and/or from decomposing Gypsum, in the
burning piles will spontaneously combine
(react) with cold or hot iron, adding
more heat to the iron, and adding
"sulfides" to the steel and
thus lowering its effective melting
temperature.
The first "molten" iron in the WTC piles
was reportedly discovered WEEKS
AFTER the collapse of the WTC towers,
and molten iron was reportedly found
regularly during the following MONTHS
during excavations of the huge piles.
The only rational explanation for this
steady-state phenomenon is IRON
BURNING. "Professor Jones" is not a
rational man, and thus he fails to
consider the fact that Iron Burns, and
instead assumes that the
reported "molten iron" was all created
(by surreptitious "Thermite") on
September 11, 2001 and that all this
red-hot liquid metal just stayed clumped
together on its chaotic descent down 70+
floors and then stayed in molten form
until it was dug up weeks and months
later.
Further, as an aside, it is total idiocy
for Jones and his associates to assume
that someone intent upon both bringing
down the WTC towers and being undetected
in doing so would go to the trouble of
actually "melting" some of the iron (let
alone allot of it) within the iron
support columns (steel will not "melt"
until reaching temperatures of nearly
3000F), rather than just heating some
of them to the much lesser temperature
point at which the iron would EXPAND
and DEFORM (see photos linked below) and
become worse than useless to support the
enormous weight of the building. (That
temperature can be scientifically
calculated given the load parameters,
and was evidently equal to or less than
the core temperature of the carbonaceous
office fires spanning an
enormous area e.g., one square acre in
size, on each of several floors of each
WTC tower). Note: "A typical house
fire can reach 2000 degrees
Fahrenheit after just five minutes of
flame."
http://www.jsc.nasa.gov/roundup/online/2004/1104_p4_7.pdf
"THE TYPICAL HOUSE FIRE REACHES A
TEMPERATURE OF APPROXIMATELY ELEVEN
HUNDRED DEGREES [Fahrenheit]"
http://www.gia.edu/newsroom/3685/broadcast_content.cfm
Aluminum melts at about 1218 F.
It is an observable fact that virtually
all carbonaceous-fires (e.g., bonfires,
house fires, burning-paper fires,
airplane fires) are readily capable of
melting aluminum. (Note: "Fire" is
not synonymous with "flame".)
When even smaller aluminum aircraft burn
on the ground, the
resulting fire usually "melts" their
aluminum portions, thus proving
temperatures exceeding 1200 degrees
Fahrenheit:
"The forward portion
of the fusilage [sic] containing the cockpit
burned, the aluminum being almost completely
consumed by the heat of the fire which
ranged from 1310 degrees to 2100 degrees
(F)."
http://www.nps.gov/yuch/Expanded/b24/b24.htm
These temperature
estimates exceed the melting point of
aluminum. See also the burning-aircraft
photos in this thoughtful rebuttal of
Professor Jones' lunatic "thermite" theory.
http://www.debunking911.com/moltensteel.htm
("Air France
flight 358 didn't hit a steel building at
500 miles an hour. It didn't even burn the
fuel in the wings yet it's aluminum skin
melted to the ground. It simply went off the
runway and caught fire. What melted the
airliner was the contents like seats,
clothing and other combustibles including
chemical oxygen generators. It's not
unreasonable to conclude the airliner and
contents didn't even need the contents of
the building to melt.") (unfortunately, the
author of that article also mistakenly
assumed that iron is "non-combustible")
[edit by Debunking911: I am correcting this with the
inclusion of this page.]
It should also
be kept in mind that "aluminum ...
ignites at relatively low temperature,"
Aluminum, "melts at about 1,220[F] degrees.
At about 1,400[F] degrees, it can
automatically ignite and burst into flames
without any spark" "The formation of
aluminum oxide is accompanied by the release
of a tremendous amount of heat ...
temperatures can reach around 5,000
degrees."
http://www.dmanuta.com/dmm/aluminum.doc
More information
about aluminum is provided here:
http://www.911myths.com/WTCTHERM.pdf
(although I think he tends to oversell the
role of melted aluminum in the collapse of
the WTC)
In other words, why use readily
DETECTIBLE "thermite" (or even
"explosives") when just burning tons of
paper, plastic, rugs, aircraft-chairs,
clothes, flesh, computers, (perhaps
aluminum metal), and some hydrocarbon
(jet) fuel, would (and evidently did)
accomplish the same result?
Professor Jones is an attention whore, who
does not check his facts:
The essay at
http://www.cagenweb.com/quarries/articles_and_books/
stone_magazine/fire_trap.html by an
early American civil engineer of great
repute ( William Sooy Smith,
1830-1916) explains the known
weaknesses of Iron (steel) beams and
columns exposed to fire. He notes that
the primary mechanism of structural
failure in steel buildings is the
DESTRUCTIVE FORCE generated in the steel
itself when it EXPANDS due to heating by
FIRE. He describes the destruction of
several steel frame buildings due to the
heat of fire, including one in New York
city. In view of these examples, there
is a warning (or prophesy) by the Fire
Chief of the City of New York of the
eventual collapse of a very tall steel
frame building, (such as the World
Trade Center buildings), due to exposure
to the heat of fire. His essay is
essential reading for anyone who would
express or consider an opinion about the
likelihood that a steel framed building
exposed to fire would be brought down by
the heat of fire.
Excerpts:
1) "Witness the Manhattan Savings
Bank building, Broadway and Bleeker
street, New York, which was
destroyed a few weeks ago by the
heat generated in the burning of
the ... building next to it."
2) "fire ... partly destroyed the
Athletic Association building in
this city. ... and it is evident
that if this heat had continued but
a little longer the whole structure
would have fallen."
3) "And notably at the burning of
the Tribune building in Minneapolis,
about three years ago, which
resulted in its entire destruction."
"There may be steel buildings in
which the fireproofing has been so
well done that they will pass
through an ordinary fire without
such failure. But if the steel
becomes even moderately heated its
stiffness will be measurably
diminished, and the strength of the
upright members so reduced as to
cause them to bend and yield. This
is more likely to occur, as the
horizontal beams and girders will at
the same time expand (unequally from
the different degrees of
temperature) and throw the posts out
of vertical and into buckling
positions. This is the third
difficulty. ... The third
difficulty, resulting from the
expansion and contraction of the
metals employed in the construction
of tall buildings, may be obviated
by protecting these metals
absolutely from any considerable
change in temperature..."
Chief Bonner, of the fire department of
New York, says in reference to the
destruction of the Manhattan Bank
building:
....We shall
have in this city, unless the
citizens of New York are warned in
time, a calamity by fire which will
rend their hearts. ... The heat
thrown from a large burning building
of any height is immense. ... I
am prepared to declare, from my
experience, that a building of brick
and yellow pine in case of fire is
easier to manage, and the contents
have more chance of being saved than
the modern fire-proof building. In
the former structure the fire burns
more slowly and has no chance to
concentrate its heat as in the iron
and steel structure.
Chief Swenie, of the Chicago fire
department, is quoted in the essay as
follows:
"I think very
much as Bonner does," said Fire
Marshal Swenie to-day, when his
attention was directed to a
statement of the chief of the New
York fire department to the effect
that the modern skyscraper is a
veritable firetrap. .... Fire in a
room so filled with goods might in
very short time gain such headway as
to imperil seriously the entire
structure by the expansion, warping
or twisting of the iron or steel
framework.
No ...
building of any kind in which
inflammable goods are stored should
ever exceed 125 feet in height, and
might with advantage be much less.
This is not because we cannot throw
water high enough. But suppose such
goods are stored in a twelve-story
building; a fire breaks out, say on
the sixth floor, and gets to burning
furiously. The heat
ascends and causes the
pillars and beams to expand.
The expansion first raises all that
part of the building above where it
takes place. At the same time the
whole weight above continues on the
expanded metal. before you know
where you are something
is going to give, and
what will be the results? They will
be too fearful to contemplate.
...
It does not take a great
amount of heat to cause steel and
iron to expand, and
when beams and columns begin moving
something has got to
break. Suppose a fire
breaks out in one of these
buildings. We work at it from below,
and the steel beams expand, the
ceiling breaks and the floor above
comes down. ...
The statements of Professor Jones and others
that "almost
no fire, even one ignited by jet fuel, can
cause structural steel to fail"
are insane distortions of reality and
misrepresentations of practical experience
of fire-fighters and engineers (See
http://www.whatreallyhappened.com/spain_fire_2005.html
A fire in a Madrid steel-frame building
collapsed 10-story sections of the building
-even without a plane crash weakening those
sections-, and almost brought down the rest
of it, which had to be torn down. "At its
peak, temperatures reached 800 degrees
Celsius (1,472 F)" ) See also:
http://enr.construction.com/images2/2006/02/060206-30A.jpg
Professor Jones' irresponsible claims
disparaging the capacity of fire to damage
and collapse iron/steel
structures are readily proved false by
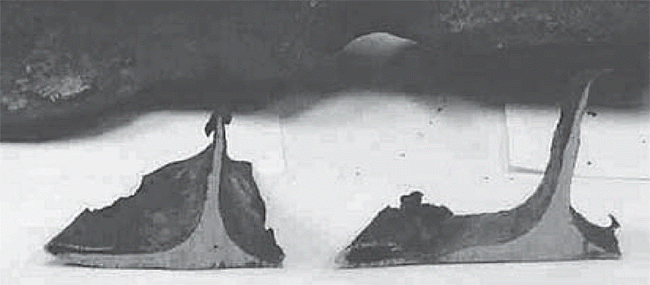
photographs of iron beams distorted and
large sections of buildings collapsed by
fire, including those photos of the
distorted iron beams in the highway bridge
that I include (below).
As for Jones' claims that a molten metal pooled
and pouring out of the floors near where the
planes impacted was necessarily iron, not
aluminum: How does Jones "get rid of" the
Molten Aluminum that would result from contact
of the airplane parts with the alleged molten
iron? Molten iron in contact with solid
aluminum will produce molten aluminum and solid
iron, or motel aluminum and molten iron (i.e.,
always molten aluminum). The molten metal
emerges (only) at the same corner and at
the same floors of the WTC where the aluminum
body of the aircraft "gently landed." What a
coincidence. Also, it almost certain that much
of the aluminum of the aircraft had melted in
the heat of the fire(s), so if "iron" can "pool"
there and pour out as Jones claims, why wouldn't
some of the tons of molten aluminum (which just
happened to land there) also pour out? What
happened to the molten aluminum according to
Jones? Jones only asserts that melted aircraft
aluminum "would
flow away from the heat source ... Thus, the
observed molten metal flowing from WTC 2 on 9/11
cannot be aluminum."
Why would melted aluminum "flow away from the
heat source" if not by action of gravity and the
shape of the surface (floors) it was pooled on?
Molten Iron would follow the same path as molten
aluminum. And, why does Jones suppose that "out
a window" is not "away from the heat source"?
Why would (pooled?) molten iron have a
preference over pooled molten aluminum to flow
"away" out of a window from the same location?
More fundamentally, what good is molten iron
falling out of a window to someone who wants to
use it to HEAT a VERTICAL IRON BEAM to the point
of failure???? In order to USE thermite to heat
something, you have to let the molten iron
transfer its heat to that thing, which means
that the molten iron would cool and solidify
if were actually USED to heat something. And,
since Jones claims that the thermite was placed
on the internal columns of the building (since
they failed first), how and why would molten
iron show up at the outside perimeter (near a
corner) to fall out of a window? Thermite
charges are always used ABOVE (or inside) the
subject to be heated, because any other position
would result in the hot molten iron formed by
thermite flowing down away from the subject to
be heated and being useless waste. Jones
offers no explanation for why anyone would go to
the trouble of using "thermite" to produce many
gallons of WASTE molten iron that was not kept
in intimate contact with vertical Beams and
therefore served no purpose other than to fall
out of a window and attract attention to
itself. So, shall we call Jones' Theory: The
Theory of the Incompetent Thermite Bombers Who
Just Needed to Call Attention to their Handiwork
by Pouring Molten Iron out of a Window. Or,
maybe the Airplanes were really Hijacked by
well-intentioned American Patriots who knew that
the only way to expose the secret plot to
destroy the WTC with Thermite was to fly a plane
into the buildings at exactly where the Thermite
was installed to hopefully cause some of its
residue to fall out a window where the World
could see it and certainly know that it was
"molten iron" produced by thermite. Bless their
souls.
Jones writes:
 
"Who can deny that liquid, molten metal existed at
the WTC disaster? The yellow color implies a
molten-metal temperature of approximately 1000
oC."
Jones admits that: "We
note that aluminum has many free electrons, so it
reflects ambient light very well -- and it appears
'silvery'. Aluminum ... aluminum would appear
silvery due to high reflectivity combined with low
emissivity..."
Look at the shiny blocky highly reflective
(silvery) solid masses that were produced from
the falling (cooling) molten metal, seen in the
bottom of the photo above right. Is it solid
Iron, or solid Aluminum?
I believe that it may be possible to "prove"
that the molten metal falling out of the WTC was
aluminum based on its behavior (e.g., breaking
up in the air, failure to "spark" white all
around, and turning into a blocky silvery solid
while falling). Aluminum is much less massive
(dense) than iron, so molten aluminum will be
more affected by air resistance than molten iron
would be. See the horizontal shift of the
falling molten metal in both of the photos
above. (E.g., Aluminum would be broken up out
of a poured stream (or blown to one side) sooner
than heavier molten iron) Also, at any given
temperature, molten iron would
probably be differently viscous or would
have different surface tension than molten
aluminum. Thus, it would visibly behave
differently upon being poured of a window. The
photos show molten metal pouring out of the WTC
that appears to be somewhat widely dispersed
(and shifted horizontally) by wind and air
resistance, suggesting that it is lighter than
iron. [It just does not quite "look" like a
stream of heavy liquid iron.] Experimentation
or simulation could prove or disprove this
hypothesis.
Keep in mind also that Jones is oblivious that
hot (molten) Iron Burns spontaneously in air.
Another problem with Jones' theory that this
falling molten metal is "iron" (and not
aluminum) is that IF it were IRON, at the
temperature of melted iron, some of it would
probably have constantly been seen
exploding/flashing/burning into bright white
Light upon being released as small particles in
the air. " The
smith's fire contains too much oxygen to allow
iron to melt; as it approaches its melting point
the iron burns instead."
http://www.osv.org/cgi-bin/CreatePDF.php?/tour/index.php?L=12&PDF=Y
Read Faraday's demonstration of moderately
heated iron particles burning in air, producing
"scintillations".
" I have here a
circular flame of spirit of wine, and with
it I am about to show you the way in which
iron burns, because it will serve very well
as a comparison between the effect produced
by air and oxygen. If I take this ring
flame, I can shake, by means of a sieve, the
fine particles of iron filings through it,
and you will see the way in which they burn.
[The lecturer here shook through the flame
some iron filings, which took fire and fell
through with beautiful scintillations.]"
http://www.fordham.edu/HALSALL/MOD/1859Faraday-forces.html
Absent constant bright White "flashes" of
burning iron droplets/particles, it more
probably was aluminum at or near its melting
temperature. I have "poured" molten aluminum
that I got by melting scrap in a
wood-fire, short distances, and that did not
readily produce flashes of light (maybe because
it cools down faster in cold air than it can
oxidize), although it theoretically can.
(molten aluminum is fairly tame) I have not
"poured" molten iron, but see this photo showing
the smaller iron droplets burning bright WHITE
in air during even a very short pouring
operation at a foundry:
And, see all the bright white sparks flying in
this series of photographs of an iron pour:
Dante observed and wrote about this commonplace
property of poured molten Iron, in his The
Divine Comedy:
"I could not endure it long, but enough to
see him sparkle all round, like
iron poured, molten, from the furnace.
And suddenly, it seemed that day was added
to day, as though He who has the power, had
equipped Heaven with a second sun."
http://www.tonykline.co.uk/PITBR/Italian/DantPar1to7.htm
Also, more definitely, the falling molten
material clearly turns into a silver colored
(highly reflective) (flat, blocky) solid
material after it cools (as soon as it stops
glowing) after falling down a number of stories
(strongly suggesting aluminum metal, not iron).
Solid iron is generally not that highly
reflective without polishing, but aluminum
is. [Molten iron would probably not loose its
glow and convert into a solid so quickly, since
it does not conduct heat as well as aluminum and
because it would be formed much hotter than
molten aluminum.
Also, iron would be expected to coalesce
into a rounder clump while falling before
solidifying. [Shot towers are used to form iron
ball-bearings, and lead musket balls, out of
poured molten metal. But, there is no
indication that aluminum can be formed into
round balls by this method, perhaps because it
cools down to quickly] If the "shot tower"
behavior of iron (forming spherical balls of
molten iron before solidifying) holds with
larger amounts of poured iron, then the molten
metal pouring out of the WTC, IF IT WERE IRON
WOULD HAVE FORMED CANON-BALL SHAPED gobs of
molten metal before it cooled down and
solidified.
The falling metal pieces formed by that
pour out the window of the WTC tower are clearly
NOT ROUND and are very elongated, or
flat, indicating a very rapid cooling of the
falling poured (aluminum) metal. [These
distinctions can be readily proved or disproved
by experimentation or calculation]. Jones
does not comment upon the silvery flat,
blocky, (not round) metal pieces visible falling
in the photo frames in his own thesis.
The NISC report seems to agree:
"The composition of the flowing material can
only be the subject of speculation, but its
behavior suggests it could have been molten
aluminum." (p. 375)
There is of course the possibility that the
falling molten metal was some other material
from the airplane or offices other than aluminum
or iron. But, I believe that there is enough
information from the video to scientifically
determine its approximate density and also its
Specific Heat, its melting/solidifying
temperature, and its thermal conductivity. The
latter determinations could be based on standard
formulas used to determine cooling rates due to
"forced convection."
"A few department chairmen at Jones'
university have issued critical statements,
though none of these has yet addressed any
of the points which Jones made in his paper
and at his presentation at BYU. Chairman of
the BYU department of Civil and
Environmental Engineering, Dr. Miller, is on
record stating in an e-mail, "I think
without exception, the
structural engineering
professors in our department are not in
agreement with the claims made by Jones in
his paper, and they don't think there is
accuracy and validity to these claims."
About Professor Jones, associated
with the so-called "9-11 Scholars"
website, I previously wrote (to
him) substantially the
following assessment of his wacky
half-baked theories about thermite
and molten iron:
Speaking as an engineer of high
academic achievement, I am
shocked that Brigham Young
University has employed an
ignorant moron of such epic and
treasonous proportions. I will
be further shocked if he is not
removed promptly from
his position of trust and
confidence. It has been my
understanding that the Latter
Day folks are a close knit group
who watch out that their members
far and wide do not embarrass
the community. In other words,
it is my hope that the Latter
Days will take the initiative to
contact the leadership at BYU so
that justice to the truth may be
served.
Excerpt of published assertions
by BYU professor Jones:
"Jones argues that
the WTC buildings did
not collapse due to
impact or fires caused
by the jets hitting the
towers but collapsed as
a result of
pre-positioned "cutter
charges." Proof, he
says, includes:
. Molten metal was
found in the
subbasements of WTC
sites weeks after 9/11;
the melting point of
structural steel is
2,750 degrees Fahrenheit
and the temperature of
jet fuel does not exceed
1,800 degrees. Molten
metal was also found in
the building known as
WTC7, although no plane
had struck it. Jones's
paper also includes a
photo of a slag of the
metal being extracted
from ground zero. The
slag, Jones argues,
could not be aluminum
from the planes because
in photographs the metal
was salmon-to-yellow-hot
temperature
(approximately 1,550 to
1,900 degrees F) "well
above the melting
temperatures of lead and
aluminum," which would
be a liquid at that
temperature.
.... No steel-frame,
high-rise buildings have
ever before or since
been brought down due to
fires. Temperatures due
to fire don't get hot
enough for buildings to
collapse, he says."
Having seen first hand the
rubble of the WTC on the night
of September 11, 2001, I can
tell you there was fire and
fires everywhere around the
scene, and fumes rose steadily
from the "piles" after the
collapse, and fumes continued to
rise from the piles when I went
back to Ground Zero over a week
later. As I described it " I
saw a hellish vapor slowly
rising everywhere from the
rubble like something out of
Dante [Inferno]." See:
While Leaving Ground Zero -
September 11, 2002
http://www.federalobserver.com/archive.php?aid=4108
(Note, I am not the same "Mark
Ferran" as the NYC fireman by
that name, and we have never
met) When I first heard about
the fires in the WTC buildings
that morning, I said to myself,
in my office, that the metal
must be getting very hot. When
I later saw the images of smoke
and fire billowing out of those
buildings, I knew they would not
stand. After they fell, the
huge piles of iron beams and
combustible materials formed two
enormous furnaces, comprising
burning office materials,
burning metal, and burning human
flesh (not to mention many tons
of combustible aircraft aluminum
and iron, i.e., thermite) which
over the course of several weeks
and months. It was widely
reported that the temperature
(e.g., measured by infra red
imaging from above) in the
interior of the piles INCREASED
in the weeks after the collapse
of the towers, due quite
obviously to the combustion
of combustible matter in these
large furnaces.
The moron employed at BYU seems
to have no conception of the
nature of a furnace, no concept
of the fact that metals
burn, and seems to be unable to
comprehend that there were much
combustible materials in the
piles from the collapsed
buildings OTHER THAN what the
airplanes brought in.
"[W]hile the jet fuel was
the catalyst for the WTC
fires, the resulting inferno
was intensified by the
combustible material inside
the buildings, including
rugs, curtains, furniture
and paper [and humans, and
aluminum of the planes].
NIST reports that pockets of
fire hit 1832°F [even before
the buildings collapsed]."
The jet fuel was the
ignition source," Williams
tells PM. "It burned for
maybe 10 minutes, and [the
towers] were still standing
in 10 minutes. It was the
rest of the stuff burning
afterward that was
responsible for the heat
transfer that eventually
brought them down."
http://www.popularmechanics.com/science/defense/1227842.html?page=4&c=y
Even ordinary dry WOOD
(charcoal) in a large enough
furnace, is capable of melting
iron:
While a mixture of aluminum and
(oxygen and iron) (e.g., rust)
called "Thermite" is capable
of producing molten
iron, evidently, a combination
of metallic Iron and Oxygen (or
Carbon Monoxide) is itself
capable of melting iron in a
large pile furnace. Large piles
of pure iron dust are capable of
"burning" themselves into a
molten mass solely due to the
heat of combustion of the iron
itself. Iron itself
is a combustible material
(and is commonly used in powder
form to warm hands and feet in
little packs sold at Wal-Mart
etc., and in MREs).
I believe that
these photos (below, and seven
images at
http://www.debunking911.com/truck.htm) fairly illustrate
the type of expansion,
distortion and yielding that
most likely happened to destroy
some of the iron columns
supporting the enormous weight
of the World Trade Centers' top
30+/- floors.

http://www.debunking911.com/alabamatruck1.jpg
The iron
columns of the WTC towers did
not "melt" in the scientific
sense of the word, but they
certainly EXPANDED (due to
heat), and yielded (due to the
enormous pressure caused by
their own thermal expansion).
Just turn these above pictures
from horizontal to vertical, and
think what would happen if that
beam were instead a column
holding up a heavy
building. (Look at the distorted
iron, heated by ordinary
hydrocarbon fuel burning, and
keep in mind what Professor
Jones said: "almost
no fire, even one ignited by jet
fuel, can cause structural steel
to fail." ) Also
note how the metal of the fuel
tank itself so completely
disintegrated. (see the
other photos at http://www.debunking911.com/truck.htm).
It's tank may have
been made of flammable aluminum
metal, like the skin and
structure of a jetliner, or of
stainless steel. I believe
that the fires confined inside
the world trade center towers
could have been much hotter than
this fairly "open air"
(unconfined) gasoline fire, due
to the greater containment of
the heat-of-combustion by the
ceilings, floors and debris in
the burning WTC towers. See http://www.zmag.org/interactive/content/display_item.cfm?itemID=3944
The False Leaders of the
so-called "9-11 Truth" movement
typically do not understand or
don't acknowledge the power of
ordinary FIRE nor the
known weaknesses and behaviors
of iron exposed to fire, and
they peddle their false
explanations of occurrences to
people even more ignorant than
them. They are the blind
leading the blind. Most of the
uneducated people (e.g.,
WebFairy,
Lisa
Guliani, Victor Thorne
etc.) selling videos books,
etc., claiming that "fire could
not have destroyed the WTC
towers" are just pathological
liars who will tell any lie to
take a buck from the gullible.
Furthermore, there is no such
thing as a "maximum temperature"
for the combustion of any dry
fuel. If you raise the
temperature of a dry fuel, like
paper, or wood paneling, or
charred flesh, and then expose
it to oxygen, its temperature
will INCREASE, not remain the
same. Duh!!! The bigger the
furnace, the higher the
temperature of the unburned fuel
gets before it combines with
oxygen, and thus still higher
will its temperature be when it
finally combusts. "Temperature"
inside of a furnace system is
solely a function of how much
heat enters the system versus
how much leaves the system, over
time, and not a function of the
type of fuel. Insulation, or a
large enough mass, slows the
exit of heat from the
system. (Note: melting things
removes energy from a system) A
large pile of debris forms an
insulating furnace retaining
much of the heat of combustion,
raising the internal
temperature, evidently high
enough to melt iron. That is
how the ancients used piles to
make and refine and melt iron
from ore.
It is shocking that a
"professor" would assume that
molten iron found weeks or
months later in the bowels of a
huge pile of continuously
burning debris (containing tons
of combustible iron and other
materials) would have to have
been generated at the very
beginning of the fire, or even
before the pile was formed. It
is even more nonsensical for him
to presume that a molten metal
supposedly formed before the
buildings collapsed would remain
molten for months without
some subsequent source of heat
being applied to it. And, it
is totally absurd for him
to presume that a molten
(liquid) metal supposedly
formed in the top floors before
the buildings collapsed (his
"thermite" theory) would remain
both molten and intact after it
fell 70+ stories in a chaotic
collapse while even more solid
objects (bones, concrete, flesh)
were obliterated on the way
down. The "professor" also
seems to be oblivious that
(aircraft) aluminum is itself a
high-energy fuel, that would not
be found in bright molten form
weeks later (because it burns
continuously when molten and
exposed to air). (They
use Aluminum metal as fuel to
propel the Space Shuttle into
Orbit around the Earth).
Also apparent, is that the
so-called "professor" is
incompetent or lazy in the use
of search engines, such as
Google. On this very subject, I
wrote this back in 2001:
"Furthermore, if it is true
that "pools of molten steel"
were found in the (basement
of) remains of the WTC twin
towers, this molten material
probably began to form and
accumulate days AFTER THE
COLLAPSE of the tower, when
the huge mass of material
trapped the heat of slowed
combustion that continued
within the pile. I saw the
fumes of combustion folks,
the piles were slowly
burning after the buildings
collapsed. Everyone with the
slightest recollection of
the events knows this. Even
a huge pile of iron filings
will form a red-hot fused
mass of metal because the
heat produced internally by
rusting will build up in the
pile. Any combustible
material in the "piles" of
the WTC that was exposed to
heat and to any amount of
infiltrating air (oxygen)
would contribute to
hot-spots. All of the
conjectures that say the
steel formed before the
buildings collapsed are
ignorant and preposterous.
The Steel in the rubble of
the WTC melted, if at all,
because of the enormous size
of the piles and presence of
much combustible materials
in them, not merely because
of the burning of jet fuel.
Those who say otherwise are
either lying, or are
overlooking something
fundamental. While jet fuel
flame burning in OPEN AIR
will may not maintain the
temperature you need to melt
steel, if you inject any
fuel mixed with air into a
huge porous mass that cannot
rapidly release the built-up
heat of combustion, you will
produce a furnace capable of
melting steel or practically
any other metal. An open
flame rapidly dissipates the
heat of combustion, but a
furnace conserves and
accumulates the heat of
combustion. Any fuel will
produce this effect in the
appropriate furnace. Its
like the difference between
the heat of an open
wood-flame of a single stick
burning in open air,
compared to the
(steel-melting) white-hot
heat produced in the bottom
of a large pile of wood and
burning wood-coals. This is
also the principle by which
large piles of organic
materials (e.g., saw-dust,
leaves, hay) will
spontaneously begin to burn-
the heat of decay builds up
inside them. "No matter
which mechanism is involved,
the oxidation reaction will
generate heat. If there is
some form of insulation,
which is usually provided by
the mass of the material
itself, the heat cannot be
dissipated. Because the heat
is not dissipated, the
temperature of the material
increases. The increase in
temperature will in turn
increase the rate at which
the oxidation reaction
occurs, which in turn will
increase the amount of heat
generated, and so on. This
increase after increase
continues until either the
heat is dissipated some way
[e.g. by melting steel], or
the material reaches its
ignition temperature and
starts to burn. (the same
basic process occurs in
stored green bio-mass
materials such as hay, saw
dust, corn cobs, etc. but
the heat is generated by the
life process of
micro-organisms)."
http://bifrost.unl.edu/ehs/ChemicalInfo/flamsol.html
"
"This scientific
principle of a furnace,
understood by primitive
humans since the bronze age,
could potentially destroy
the credibility of anyone
who forwards and endorses
the erroneous theories
(e.g., "nuclear" bombs). You
are literally playing with
fire by promoting such bogus
theories. People, for the
sake of our country, and out
of respect for those who
died at the WTC, please do
not promote or forward those
Urban Legends.
"I am sorry if my words
are harsh, but I do not have
much patience for people who
are either irresponsible for
forgetting what they
themselves saw, who pretend
to understand physical
principles that they have
not studied or otherwise
have no competence in, or
who are simply liars who are
out to make a reputation by
misrepresenting to others
what happened on September
11, 2001. Everyone with
common sense knows that two
commercial air planes hit
and burned inside the towers
and caused the towers to
break and to fall. Mark R.
Ferran BSEE scl JD mcl
http://billstclair.com/ferran/index.html
I am aware that there are
millions of science-ignorant
people and some total morons
walking around America babbling
about the World Trade Center
(and I have tried in vein to
address this http://www.zmag.org/interactive/content/display_item.cfm?itemID=3944
) , but when a "professor" who
knows that he has no formal
education nor any
practical education in the
science of chemistry,
combustion, nor of metallurgy,
nor of the Strength of materials
decides to spew his ignorant
reckless notions as
scientific "FACTS" to the
gullible volatile public at a
time of crisis, I feel that his
reckless conduct warrants
extreme and swift
punishment. Professor Jones has
also misrepresented the
significance of the "Law of
Entropy" to bolster his false
claims. Given the tendency of
this
professor's misrepresentations
to give aide, comfort, and
encouragement to those who have
overtly declared Jihad against
our pathetic country, (and who
must be able to recruit more
jihadists just by laughing at
our domestic morons) I would be
satisfied to see this
"professor" tried, convicted,
and executed for Treason. He
breached a Trust in time of
WAR. Jones' reckless remarks
will probably kill as many
Americans as President Bush's
misuse of the word "Crusade" has
and will.
I think it is preposterous for
anyone to assume that a
tall building or any
conventional material or mode of
construction can not fall down
if you fly a large fuel-laden
airplane into it at more than
500 miles per hour.
In summary, we have a
moron posing as President, and
now we have morons posing as
"Professors" too. No wonder
that the people of the world
increasingly find it necessary
to destroy US for their own
preservation.
Mark Ferran BSEE scl JD
mcl
P.S.
Snopes may be a good starting
point for information to counter
some of the Anti-American
9-11 propaganda (e.g., from the
French):
Sent: Friday, July 14, 2006 7:01 PM
Subject: Re: Professor Jones: WTC
IRON BURNS!!! updated July, 14 2006
Professor Jones of
BYU has since acknowledged in an
email to me the fact that "iron
burns" but attempts to limit
this chemical reaction to only
"under certain conditions" (he
specifies pure oxygen as supposedly
being a requirement), but Jones has
failed to acknowledge that hot Iron
is combustible (it oxidizes and
generates heat) when combined with
ordinary air, and Jones has not
responded to the
following observations that iron is
usually sulfidated upon exposure to
the fumes from ordinary fires.
----- Original Message -----
Sent: Friday, July 14, 2006 7:54 PM
Subject: Re: Professor Jones: WTC IRON
BURNS!!! updated July, 14 2006
MARK: Now we are getting somewhere. Obviously
iron burns at some temperature. At what
temperature does iron reach the condition at
which it burns and at what temperature does
structural steel, which is what was used in the
construction of the WTC buildings, reach the
condition at which it burns? Is the temperature
for the condition which causes the burning of
structural steel higher than that of iron? What
American Society Of Civil Engineers (ASCE)
specification did the structural steel used in
the construction of the WTC buildings meet? Did
the specifications meet the requirements of the
American Institute for Steel Construction
(AISC)?
At what temperature does jet fuel burn?
KEEP up the good work Mark. With a name like
Ferran, we can expect the best information about
ferro!-----BILL
To: William
Cc: Kevin Barrett
Sent: Saturday, July 15, 2006 12:37 AM
Subject: Re: Professor Jones: WTC IRON
BURNS!!! updated July, 14 2006
William, you asked these
innocent questions:
"Obviously iron burns at some
temperature. At what temperature does iron reach
the condition at which it burns and at what
temperature does structural steel, which is what was
used in the construction of the WTC buildings, reach
the condition at which it burns? Is the temperature
for the condition which causes the burning of
structural steel higher than that of iron?"
Fe + O2 -> FOx plus heat.
For example,
4 Fe(s) + 3 O2(g)
----> 2 Fe2O3 (s)
Delta Go =
1487 kJ
Iron wire ("Steel Wool"),
which has a coating of oil to prevent rusting, will
"ignite" and quickly transform the fine iron wire into
iron oxide(s), with just the touch of the small flame of
a match. So, the activation energy/temperature for
fast iron oxidation is not very high relative to the
heat of an ordinary flame fire.
"rusting (oxidation of
iron) is overall very exoenergetic. Even though
under normal conditions the oxidation reaction
proceeds very slowly, there is sufficient energy to
initiate the process at room temperature. Once
begun, the process can sustain and slightly
accelerate itself by using the heat released from
the reaction and that initially available from
room-temperature to provide the necessary activation
energy."
http://www.newton.dep.anl.gov/askasci/chem03/chem03046.htm
And,
"'Rust' is really a
family of iron/oxygen compounds with various ratios
of: Fe, O, and H (as in water). All the 'rusting'
reactions are exothermic to the tune of from about
-60 to -190 kcal/mol That is they all liberate a
substantial amount of heat. In fact one can
generalize that for most metals oxidation is
exothermic. Now the "activation" energy to which you
refer has to do with just how "fast" the reaction
occurs. In the case of iron. I do not have the
numbers in front of me but from qualitative
observation I would expect it is quite small...."
http://www.newton.dep.anl.gov/askasci/chem03/chem03046.htm
Impurities, (e.g., water,
salt) and even some bacteria may promote or
catalyze iron oxidation (rust) at room temperature. You
generally cannot prevent room-temperature iron surfaces
exposed to the environment from oxidizing in air except
by painting it, coating it (e.g., galvanizing), or
combining it with something else (e.g., adding chromium
in to make stainless steel). Structural Steel is not
"stainless steel" and therefore it is chemically
reactive the same as pure iron, for purposes of
oxidation by air.
Pure Iron oxidizes fairly
rapidly at "hot" temperatures. The hotter it is, the
faster an exposed hot iron surface will react (oxidize)
in air, generating more heat. The iron-oxidation
reaction and resulting reactive surface temperature may
be further increased by adding pure oxygen, but that is
not necessary to sustain exothermic (i.e.,
heat-producing) iron oxidation. To get the reactive
surface of a mass of rapidly oxidizing iron to
incandesce bright white you probably have to add pure
oxygen, which will raise the iron-oxidation reaction
rate at the surface, and raise the temperature at the
surface, etc.. Some people would identify this
incandescing phenomena as "burning" iron/steel. But,
even while not incandescing white, red-hot iron exposed
to air is "burning", just somewhat slower.
Red-hot iron exposed to air
IS OXIDIZING, (or "burning"), the only question being
"how fast" is it "burning". In a big deep pile like the
debris piles of the WTC buildings, the iron-oxidation
reaction will probably be moderated by the availability
of atmospheric oxygen. In other words, the reaction
will take place at the rate at which oxygen from
air infiltrates into that part of the pile (e.g., by
convection). [Note that SO2, e.g., generated by Gypsum
decomposing in the piles will also cause additional
iron-oxidation, plus sulfidation] However, the
resulting change in temperature is a function of how
much heat is generated minus how much heat is lost by
convection, conduction, and radiation within a period of
time. Deep inside the piles these losses are relatively
small, and the larger the scale of the red-hot
iron-fire, the smaller these losses are on a per mass
basis. See http://www.tcforensic.com.au/docs/uts/essay6.pdf#search='
temperature%20iron%20combustion'
Professor Jones himself
expressly notes that the pile has "insulating"
properties and would conserve the heat of oxidation, but
Jones overlooks that it is primarily hot IRON that will
be oxidizing weeks and months after the collapse.
You asked "What
American Society Of Civil Engineers (ASCE) specification
did the structural steel used in the construction of the
WTC buildings meet? Did the specifications meet the
requirements of the American Institute for Steel
Construction (AISC)?" as if these specifications or
requirements were somehow related to the oxidation
(burning) of hot structural steel members.
The ASTM or ASCE or
AISC standards do not have
anything to do with the chemical reaction between hot
iron and oxygen.
The AISC does not certify
individual pieces or batches or structural steel. It
only ANNUALLY certifies the methods and procedures of
the manufacturer:
Kevin Ryan committed
deception and was properly fired. Kevin Ryan
falsely asserted:
"We know that the steel
components were certified to ASTM E119. The time
temperature curves for this standard require the
samples to be exposed to temperatures around 2000F
for several hours. And as we all agree, the steel
applied met those specifications."
I looked up what the ASTM
E119 standard actually is. ASTM E119 does NOT test
"steel" nor "steel components" per se as
Mr. Ryan had implied. Rather, ASTM E119
time-temperature tests evaluate whole building
assemblies that include fire-proofing or
fire-resistance:
"ASTM E119, Standard Test Method
for Fire Tests of Building Construction and
Materials, is used to determine the fire resistance
of a complete assembly. For example, a wall system
fire rating is measured by constructing a 10 foot by
10 foot section of a total wall system: framing,
cavity insulation, sheathing, siding, gypsum wall
board, etc. The wall section is installed vertically
on a gas furnace, and the wall is exposed to a
standard temperature curve for the time period for
which a rating is desired, i.e., one, two, three, or
four hours. Failure points during time of fire
exposure are:
"• Flame penetration through the wall section;
"• An unacceptable temperature increase on the
unexposed side of the assembly;
"• Structural failure or collapse of the assembly.
"Therefore, a one hour fire resistance rating is
taken to mean that a structure incorporating the
tested wall construction will not collapse, nor
transmit flame or a high temperature, while
supporting a design load, for at least one hour
after a fully developed building fire."
http://www.pima.org/technical_bulletins/tbull105.html
The chemical and physical or
thermal properties of the framing steel members are
standardized and known, or are tabulated in catalogues,
and determining such are not the object of the ASTM E119
testing. Rather, it is the functionality of the
fire-proofing or fire-resistance of the whole assembly
that is tested. After you crash an airplane into a
building, the ASTM E119 test results become totally
irrelevant, because you have changed the structure, at
least by removing the fire-proofing or the
fire-resistant wall and ceiling materials. The ASTM
E119 certification is intended to estimate how long
the structural steel WILL BE PROTECTED FROM EXPOSURE to
temperatures around 2000F.
There is no "structural steel"
column that is going to continue to support the weight
of a heavy tall building after being directly "exposed
to temperatures around 2000F for several hours". ASTM
E119 certification does not purport to impart such super
heat-resistant properties to structural steel. See also
Kevin Ryan's own words indicate
that he really knew nothing at all about the ASTM
E119 testing that his "company" supposedly performed
on WTC "steel", and that he had no right to
misrepresent it's significance. Hence, he was
incompetent and presumptuous, and he deserved to be
fired, or even sued for fraud, or even criminally
punished.
The only thing Kevin Ryan was
certainly right about is that structural steel in
the standing WTC buildings did not actually "melt"
(to a molten liquid) before the collapse of the WTC
buildings. It did not have to. "It does
not take a great amount of heat to cause steel and
iron to expand, and when beams and
columns begin moving something has got to
break." http://www.cagenweb.com/quarries/
articles_and_books/stone_magazine/fire_trap.html
However, at least some of the
aluminum alloy metal from the crashed airplanes
certainly did actually melt before the collapse, and
that indicates a temperature of about 1,200F in the
infernos at the crash sites.
You ask, "At what temperature does jet
fuel burn?" as if that were a meaningful question.
The answer(s) depend upon what the
temperature of the Jet Fuel is at the moment that it
combines with oxygen, and upon the temperature of the
environment that it is burning in. I have a now-rare
device called a Kerosene flame-thrower that I used to
use to crack/shatter hard rocks and melt glass just
because it's flame was hot enough, and because it was
fun.
There is a coil in the hot end that
transports and pre-heats the kerosene before it is
released out the flame nozzle through the coil.
If you had just poured the same kerosene
onto a rag and lit it up, it would not burn as hot as
the flame coming out of the nozzle of that burner.
If you construct a large enough furnace
in which the temperature of the liquid fuel itself will
be increased to its boiling point and then further heat
the fuel vapor before exposing it to air, then there is
no limit upon the temperature that may be attained by
burning kerosene (Jet fuel) except that the fuel will
decompose into carbon at some high temperature (and thus
cease to be that fuel). A pure-carbon (charcoal) fire
is very capable of melting steel. If you super-heat jet
fuel, you can get pure carbon and hydrogen anyway. So,
depending on the size and configuration of the furnace,
you can melt iron with jet fuel. I do not believe
that flaming jet fuel literally melted any iron in the
WTC towers. No one with any intelligence does.
Mark Ferran BSEE scl JD mcl
P.S. Thank's for noting that my family's
name is derived from the Latin Root word for Iron, Ferr,
Ferro, Ferris. You are the first to notice that.
From: William
Cc: Kevin
Barrett
Sent:
Saturday, July 15, 2006 6:33 PM
Subject:
GETTING DOWN TO BRASS TACKS
MARK: First, I want to thank you
for responding forthrightly
without the accompanying
characterizations ( although
"innocent questions" might fall
into that category).
The
terms oxidizing and burning, in
my mind, represent two states of
the oxidation process. There is
the slow oxidation process at
relatively low temperatures,
such as the oxidation of copper
roofs or gutters or
flashing which produces a
coating on the copper that is
green or the oxidation of
grandma's family silverware
which produces a coating on the
silver that is gray. There
is even a special alloy
steel called Corten steel that
produces a protective rust
coating that serves as a barrier
to further oxidation and
therefore does not require
painting to preserve its
integrity. The Ford Foundation
Building in New York City
used exposed Corten steel as an
architectural enhancement.
The
state of oxidation of the
examples cited is distinctly
different from the oxidation
that takes place with the fire
of fuel oil in the basement
furnace or of the wood in the
ski lodge fire place or of
the fire from propane gas used
to solder the copper pipes. This
different state of oxidation is
like the different states of
water as liquid, or as a solid
ice, or as a gas steam--- they
are all water but in a different
state that require different
temperatures to produce the
different states.
Burning, therefore, is the state
of oxidation that occurs at the
time that ignition temperature
is reached which is generally
when the temperature is
sufficiently high to transform
the oxidizing material into a
gaseous state. It is an ignition
temperature which increases with
the increase in the mass of the
oxidizing material. The ignition
temperature would be lower, for
instance, to ignite steel wool
than the temperature required to
ignite the three inch
thick steel column flanges used
in the World Trade Center Twin
Towers. To prove my point, after
igniting your steel wool with a
match try using a match to
ignite your screw driver.
Furthermore, the product of the
oxidation that produces ferrous
oxide, or copper oxide, or
silver oxide is completely
different than the product of
burning which produces carbon.
So,
to clarify or to be more
precise with the questions I
asked-------
What
is the ignition temperature of
iron and what is the ignition
temperature of the steel
that was used in the
construction of the WTC
buildings? Is the ignition
temperature of structural steel
higher than the ignition
temperature of iron?
What
is the temperature required to
cause iron to begin to become
flexible or steel to begin to
become flexible? How long would
that temperature have to be
sustained to create the flexible
condition in the two or three
inch flange of a structural
steel column of the Twin Towers?
What
is the burning temperature of
Jet fuel?
In
this respect, the American
Society for Testing Materials
test 119, ASTM119, does not test
for the ignition temperature of
structural steel but, as you
have correctly shown, for
testing a protective coating or
assembly. For instance, a three
hour rated wall used in the
construction of the stairwell of
a hi-rise apartment building is
for the purpose of providing
protection for three hours in
the enclosed stairwell. The same
is true for a three hour rated
door used to access the
stairwell.
BILL
To: William
CC: ; Kevin Barrett
Sent: Saturday, July 15, 2006
9:01 PM
Subject: Re: Burning Iron and
Jet Fuel
My response
was geared towards someone who I had
supposed to be completely
unknowledgeable, and was thus
simplistic, and the question did not
mention "ignition" and so the response
did not get into a discussion of tipping
point of "ignition" temperatures. The
only relevant point is that oxidation
takes place on a red-hot surface of
iron/steel. It does not matter that
such red-hot metal is not oxidizing
rapidly enough "to transform the
oxidizing material into a gaseous
state", and to thus produce
incandescence.
It is not
necessary that the "oxidation"
occurring that
the surface reaches the tipping point of
"ignition" at which the temperature
rises abruptly due to the
rate-of-oxidation (rate of local heat
generation) exceeding the rate of
heat-dissipation. PRIOR TO THAT POINT,
heat-generating oxidation is already
taking place. If you somehow shave
off a razor-thin slice of a red-hot
block of iron, (the only thing changed
being the ratio of mass to surface area
of the shaving) the shaved slice will
likely ignite and incandesce just
like burning "steel wool" because of the
change of the mass associated with its
reactive surface area (also doubling the
surface area). The initial rate of
oxidation at the surface at that red-hot
temperature is not different. What
changes is that the heat already being
generated by oxidation at the surface is
not drawn away by conduction into the
larger mass (and is doubled). So,
while it is true that there is a point
of "ignition" (with incandescence) of
iron, such ignition is CAUSED BY the
heat generated by the slower oxidation
already taking place at that surface.
Ignition (oxidation at a rate
sufficiently high to transform the
oxidizing material into a gaseous
state) is not the point at which
oxidation begins. The loss of heat
generated by oxidation at the surface is
dependent upon conduction (which depends
upon the mass of the material
heat-sink), and upon radiation and
convection (both of which depend
inversely upon ambient temperature).
So, when you increase the mass, you most
compensate by increasing the ambient
temperature. And this is why: "ignition
temperature .. increases with the
increase in the mass of the oxidizing
material".
When you are
oxidizing a large tangle of iron masses
in a large pile furnace, and the iron
masses and the ambient gases and the
neighboring materials are all at
approximately the same temperature,
there are minimal losses of heat from
the whole SYSTEM, so the temperature of
the whole system gradually increases
with continued oxidation of the iron,
even though there is no incandescence
(no very high rate of oxidation). [the
temperatures of the iron masses and the
ambient gases and the neighboring
materials will continuously equalize as
they increase together]
Your additional questions concerning
"What is the ignition temperature of
iron" and its structural alloy
(steel) are purely academic SINCE: IF
that "ignition" point is defined as the
ambient temperature at which the
oxidation of iron is fast enough to heat
the iron's surface temperature high
enough "to transform the oxidizing
material into a gaseous state", THEN an
"ignition" (incandescence) point was
probably not attained within the burning
debris piles at WTC. There is no
evidence that oxidation of steel
(iron) in the piles proceeded fast
enough "to transform the oxidizing
material into a gaseous state" with
attendant white incandescence.
And
differences between the theoretical
ignition points of pure iron and alloys
of iron (steel) are likewise academic
and immaterial to the discussion of
"molten steel". Professor Jones does
not claim that there was "gaseous steel"
in the piles, and there is no evidence
of it. Neither do I.
Most
people use the term "burning" to denote
the point at which oxidation becomes
noticeable to them, such as the point of
incandescence. However,
materials at temperatures that are below
their "ignition" (incandescent burning)
temperature will still be oxidizing.
That is why a pile of oily rags (or a
pile of sawdust) can increase its
internal temperature and "spontaneously
combust" with flames. If there had
been no slower oxidation prior to the
visible manifestations of combustion
(e.g., smoke and flames) there
ultimately would have been no
spontaneous combustion.
So,
"oxidation" is the proper terminology to
describe both slow oxidation
(rusting) and medium oxidation (hot, but
sub-ignition) and very fast oxidation
(ignition with incandescence).
All
"burning" is "oxidation."
All
"combustion" is "oxidation". Arguably,
all "oxidation" is "combustion".
It is an immaterial
and unnecessary semantical debate
whether all "oxidation" including very
slow oxidation (e.g., rusting) is
"burning." I do not believe that
the acceptable usages of "burning"
necessarily requires that the rate of
oxidation "is sufficiently high
to transform the oxidizing material into
a gaseous state." Burning pure carbon,
e.g., graphite, or carbon soot, which
definitely "burns" in air, does not
produce "flames", and does not produce
at its surface any carbon in a "gaseous
state." The temperature at which pure
carbon turns into a "Gas" is a
super-high temperature: Carbon
boils/vaporizes at about 4500
K. "Pure carbon boils at 4300
K"
http://www.extrasolar.net/forums/viewtopic.php?t=60 (Iron
boils at "2450 K" http://www.physics.usyd.edu.au/teach_res/db/d0005f.htm )
Thus, although pure carbon "burns" in
air, it does not have to reach its
vaporization (gaseous) temperature to do
so. An ordinary wood fire burns down
to charcoal (after burning off the
volatile constituents of living wood)
that can burn red-hot without producing
any visible gaseous "flames". Neither
gaseous carbon nor flames are necessary
for "fire" or "burning" to be
occurring. Neither
gaseous iron nor flames are necessary
for "fire" or "burning" to be occurring.
and there is no point in using it or
stainless steel for internal structural
elements, since, structural elements
are supposed to be protected from fire
and from corrosive elements.
See
You now ask
questions that appears to be entirely
different from the subject of what
burned in the debris piles after the
collapses:
"What is
the temperature required to cause
iron to begin to become flexible or
steel to begin to become flexible?
How long would that temperature have
to be sustained to create the
flexible condition in the two or
three inch flange of a structural
steel column of the Twin Towers?"
I think you
need to provide much more context for
these questions to be meaningful.
Your question seems to assume that the
force or pressure on the steel is
immaterial to the answer. This is not
the case. Steel at room temperature is
somewhat "flexible" given enough force
or pressure being applied to distort
it. Press or strike a room-temperature
I-beam hard enough, and it will dent or
deform, not shatter. Unlike cast iron
or pure iron Structural Steel ordinarily
does not "shatter" when it cannot
support a load without deforming.
Your
questions also seem to assume
that a "temperature required to
cause iron to begin to become
flexible or steel to begin to become
flexible" must be maintained for
some extended period of "time" in
order "to create the flexible
condition". That is not correct.
The elastic properties of iron or
steel are generally temperature
dependent, not time-temperature
dependent.
The
Young modulus for steel is
temperature-dependent:
When
the steel is AT a certain
temperature, it has the associated
physical properties (e.g., Young
modulus) while and for as long as it
is AT that temperature (unless the
temperature is high enough to alter
the microcrystalline structure of
the iron). So, assuming no change
to the microcrystalline structure of
the steel ("The
mechanical properties of steel
are dependent on its microstructure"),
the literal answer is Zero Time.
Note: It
is an entirely different question
how much time it takes to heat up a
mass of steel TO such a given
temperature. That question is also
totally context-dependent.
All 9-11
internet-published conspiracy
theorists, that I have read, are
unaware that long before steel
members subjected to the heat of
fire lose much of their
rated strength and rigidity (before
their Young modulus decreases too
much), they EXPAND in a manner that
creates a force that can destroy
them, or destroy other members, or
destroy their connections to other
members. The combination of thermal
expansion with a still-high Young
modulus creates an enormous force,
which typically causes something to
distort, tear off, or to move out of
the way. As they said back in the
day, "something has got
to break."
http://www.cagenweb.com/quarries/articles_and_books/
stone_magazine/fire_trap.html
Particularly, which "two or three inch
flange of a structural steel column" are
you referring to, and what is it holding
up? floor trusses ends? And, why do you
suspect that such a flange became too
"flexible"?
Again you
ask "What is the burning temperature of
Jet fuel?" without providing the context
in which it is burning. Whatever
context, such a temperature is likely
comparable to the burning temperature of
other hydrocarbon fuels, such fuel oil
or gasoline, or diesel fuel.

The
hydrocarbon fire (gas, diesel, oil)
whatever it was, caused the steel I-beam
above it expand, and where the beam was
"softest" (its Young's modulus was
reduced where it was heated) the beam
was deformed by that force. The beam
could not move the objects pinning its
ends (could not freely elongate), so a
compressive distortion occurred within
the length of the beam itself. Upon
cooling, the beam did not resume its
original shape, nor its original length,
because the forces generated
by its thermal expansion had
compressively deformed it. If this were
a vertical column supporting a heavy
building in parallel with other columns
(which would restrain it from freely
elongating), the column so heated and
deformed would not be able to carry its
rated load, and UPON COOLING SOME it
would then thermally contract but would
be x inches SHORTER than when it was
installed and thus it could be PULLING
THE BUILDING DOWN INSTEAD OF HOLDING IT
UP.
So, to
answer your question, "What is the
burning temperature of Jet fuel?", I
would say that the burning temperature
of Jet fuel is about the same
temperature needed to cause installed
structural steel members to expand and
to distort, and to potentially
change their shape, position, and/or
length.
|

http://www.answers.com/topic/steven-e-jones linked from http://reddit.com/info/48t1/comments