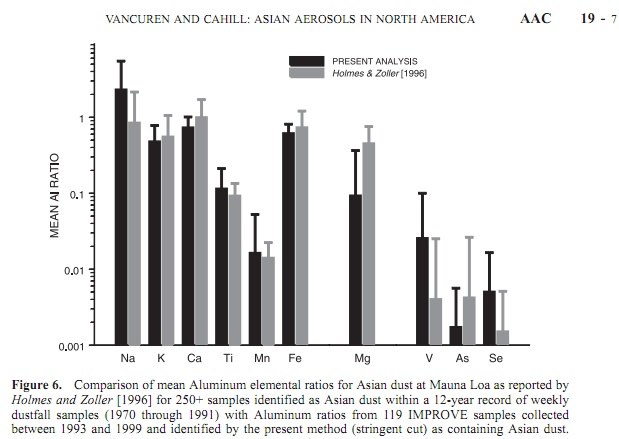
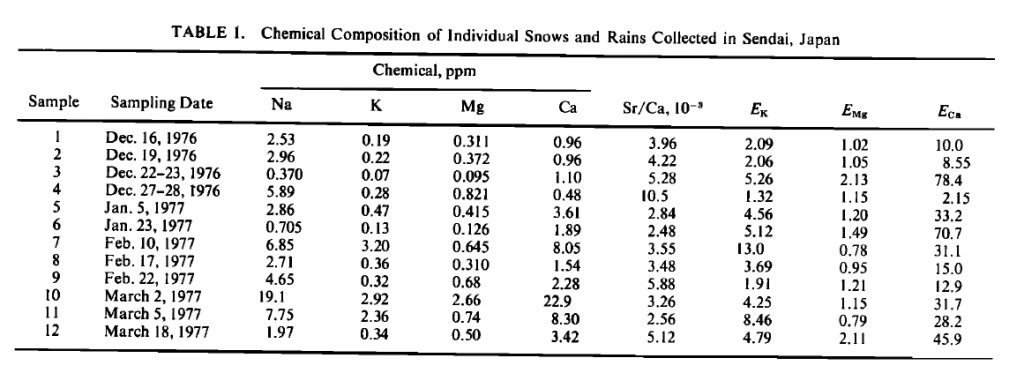
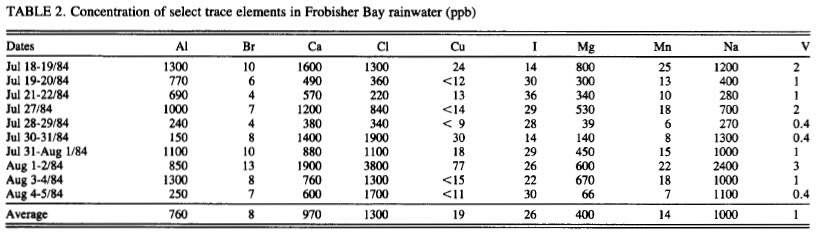
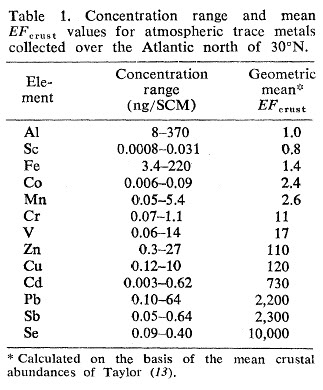
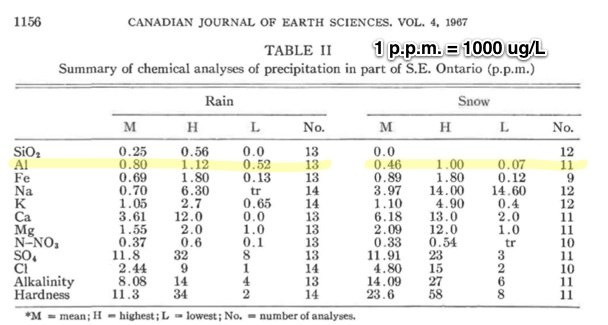
These figures of the concentration of aluminum in rain and snow match WITWATS/Mangels figures quite well, 520 ug/l to 1120 ug/L

A PRELIMINARY STUDY OF THE COMPOSITION OF PRECIPITATION IN S.E. ONTARIO
G. K. RUTHERFORD
Department of Geography, Queen's University, Kingston, Ontario, Canada
Received April 14, 1967
As a prelude to the study of chemical weathering of rocks and soils in S.E. Ontario, a study of the composition of precipitation waters at 12 sites within the area was initiated. Analyses of bulk precipitation of both rainfall and snowfall are presented and compared with similarly derived results in other areas.
1 he silica and aluminum contents are regarded as being high, although in snow silica is absent and aluminum is quite high. Areal differences in silica content appear to be connected with local differences in lithology. Iron occurs in the same concentrations as aluminum and is probably derived from atmospheric dusts. The sodium content is high, especially when compared with inland sites in other areas. Potassium is generally somewhat lower than sodium. Although the calcium content may reflect local differences in lithology, the dissolved magnesium is surprisingly uniform. Lithological differences in tne area are also reflected in the significantly higher nitrate-nitrogen concentrations in the limestone area. Snowfall is markedly high in sulfates, which is considered to be a function of climatic and industrial causes. The high chloride concentrations in such a 'non-marine' environment are not explained.
...
Reliability of the Data
The samples contained dry fall-out and dust settling on the gauges so that they were filtered before examination in order to remove the undissolved particles. The overall accuracy of the data is difficult to assess. Microbial activity in the water samples can alter chemical concentrations of nitrates, but has much less effect on other constituents. Large integrated samples of pre- cipitation can give no more than an average for the period concerned. It is realized that the figures presented give only general indications of ionic levels in precipitation, and a more accurate picture will emerge when sampling is more frequent. For reasons stated above, the comparison of these results with those
of other workers must be considered with caution. As far as possible, com- parisons will be made with analyses of bulk precipitation where this is known for certain. However, with respect to soil weathering, the analyses of rainwater should be more relevant than those of bulk precipitation, although the differ- ence may be hardly significant.
The literature has an unexpected paucity of analytical results for the con- tents of Al and Fe rainwater and snow. In fact, no data for Fe could be found and only the results of Feth et al. (1964) for Al in snow could be brought to light.
...
Aluminum
As aluminum is geochemically stable, the figures for this element in Table I must be considered high for both snow and rain. The aluminum content of rainfall is similar to silica but significantly higher for snow. The figures are of the same order of magnitude as those recorded for the St. Lawrence River (Clarke 1924), for lake and river water (Rankama and Sahama 1949), and for snow (Feth etal. 1964).
Attachments
Last edited: