GrandVizor
New Member
Lectori salutem,
I'm from the Netherlands, Europe.
Excuse me if my English is not 100% correct.
I'm in a debate with a lot of chemtrail believers in which I am the scepticus.
I use this website as a resource for a few months now and I thank you all for the helpful explanations and references.
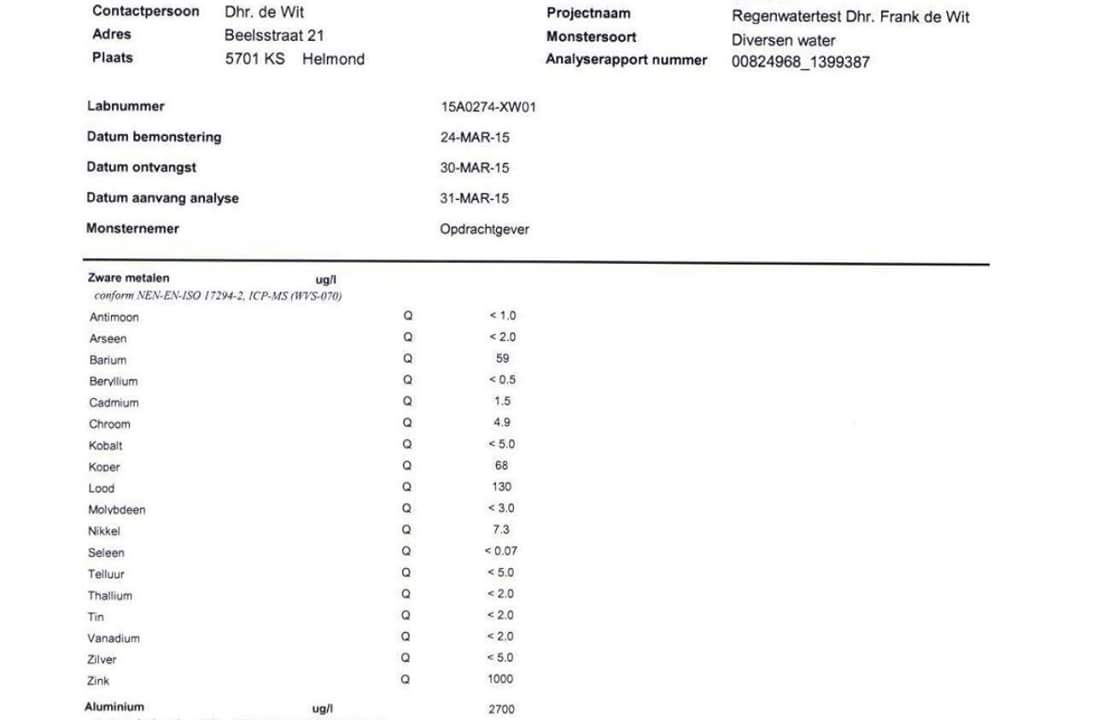
Recently a rainwater test result popped up and I need to know what the acceptable value for Aluminium is. I've looked around for it and learned a lot about it, but the answer is still inconclusive to me.
In will enclose a picture of the results for your assessment.
I thank you in advance for any provided help in this matter.
The Aluminum level is 2700 µg/l
I'm from the Netherlands, Europe.
Excuse me if my English is not 100% correct.
I'm in a debate with a lot of chemtrail believers in which I am the scepticus.
I use this website as a resource for a few months now and I thank you all for the helpful explanations and references.
Recently a rainwater test result popped up and I need to know what the acceptable value for Aluminium is. I've looked around for it and learned a lot about it, but the answer is still inconclusive to me.
In will enclose a picture of the results for your assessment.
I thank you in advance for any provided help in this matter.
The Aluminum level is 2700 µg/l
Last edited by a moderator: